Introduction: The Next Big Shift in Energy Storage
For decades, the lithium-ion battery has dominated the energy storage market—powering everything from smartphones and laptops to electric vehicles and renewable energy grids. But as demand soars, the limitations of lithium are becoming hard to ignore: rising costs, limited reserves, and the environmental impact of mining. This is where the sodium ion battery steps into the spotlight.
Unlike lithium, sodium is one of the most abundant elements on Earth, found in everyday salt and available almost everywhere. The push for clean energy and sustainable electrification has accelerated research into sodium-based storage solutions. In fact, many scientists and companies are now debating sodium ion battery vs lithium ion technology as the race for the next-generation battery intensifies.

The sodium ion battery could represent the next big leap, offering a cheaper, more sustainable, and widely available alternative. While lithium will likely remain important, the growing momentum behind sodium ion battery vs lithium ion discussions suggests that the energy future may look very different within the next decade.
What is a Sodium-Ion Battery?
A sodium ion battery is an energy storage device that works much like a lithium-ion battery but uses sodium ions instead of lithium ions as charge carriers. In simple terms, the battery stores and releases energy by moving sodium ions back and forth between the electrodes during charging and discharging.
The fundamental advantage of the sodium ion battery lies in its raw material: sodium. Unlike lithium, which is concentrated in a few regions of the world and subject to supply chain risks, sodium is cheap, non-toxic, and abundantly available in seawater and the Earth’s crust. This makes the sodium ion battery a highly attractive candidate for large-scale applications like renewable energy storage and affordable electric vehicles.
Another important distinction is size: sodium ions are larger than lithium ions, which affects energy density. While a sodium ion battery may not yet match the compact performance of lithium for small electronics, its scalability, low cost, and sustainability make it a strong contender for the next wave of global electrification.
Sodium-Ion Battery: Construction and Working
A sodium-ion battery is built from the same basic parts as a lithium-ion cell, but every component is chosen and optimized for sodium chemistry. Understanding sodium ion battery construction and working means looking at the electrodes, electrolyte, separator and the way sodium ions move during charge and discharge.
Main components
Anode (negative electrode)
- Most commercial sodium-ion cells use hard carbon as the anode material because graphite (used in lithium cells) does not intercalate sodium ions efficiently. Hard carbon can accommodate larger Na⁺ ions and offers a good balance of capacity and stability.
- Research also explores titanium-based or other alloy/oxide anodes that can host sodium reversibly; these are attractive for fast charging and long cycle life but often trade off capacity.
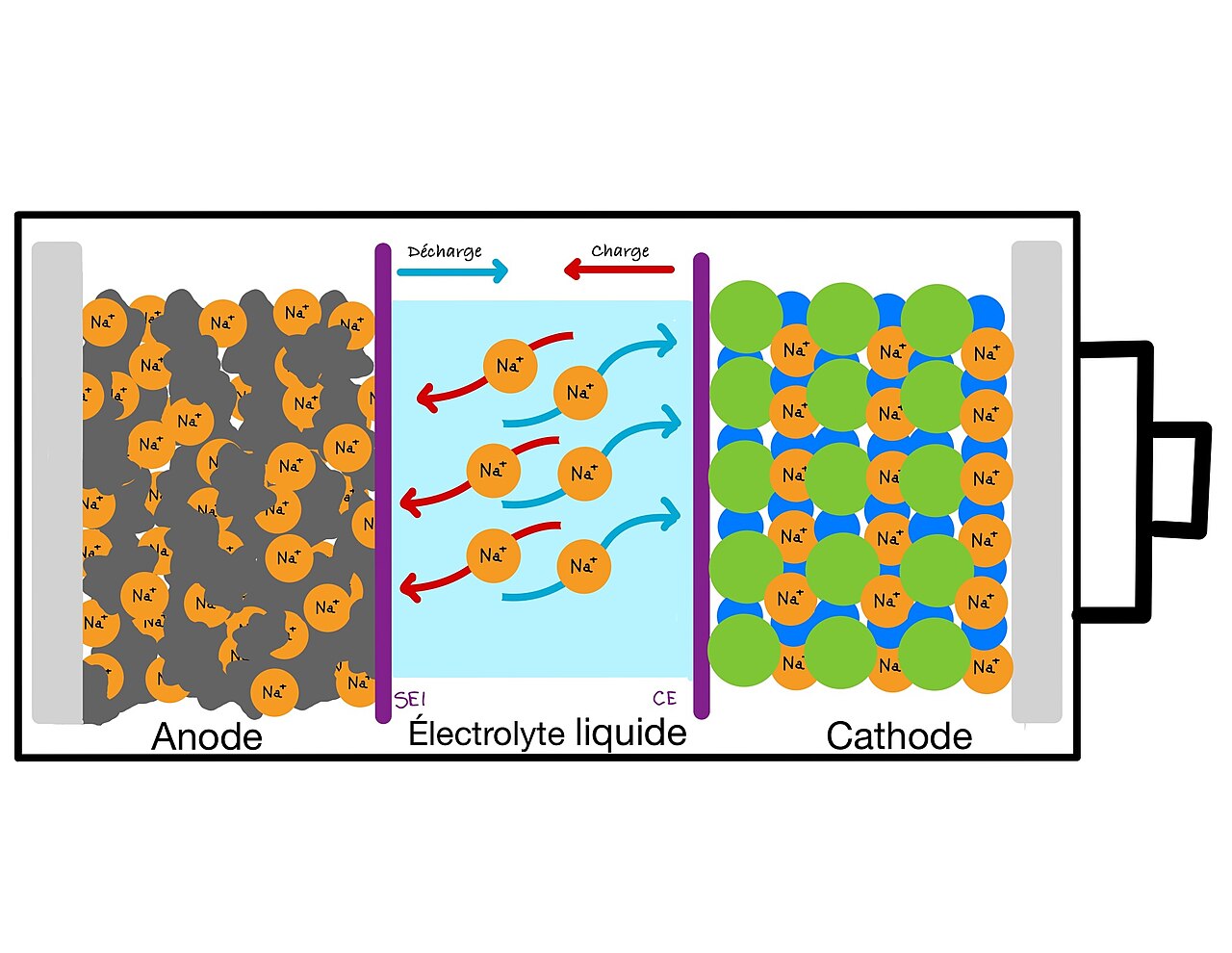
Cathode (positive electrode)
- Common cathodes are layered sodium transition-metal oxides (often written NaxMO2, where M = Ni, Mn, Fe, Co combinations) and polyanionic compounds (NASICON-type, phosphates such as Na3V2(PO4)3 or NaFePO4 variants).
- Polyanionic cathodes usually offer excellent thermal stability and safety; layered oxides typically give higher voltage and energy density.
Electrolyte
- Liquid organic electrolytes with sodium salts (for example, NaPF6 in carbonate solvents) are common in lab and early commercial cells. Alternatives include ionic-liquid electrolytes, ether-based solvents, and solid electrolytes (NASICON-type ceramics) for solid-state sodium batteries.
Separator & cell stack
- A porous polymer separator keeps electrodes apart while allowing Na⁺ to pass. The electrodes are coated on current collectors and stacked/rolled to make pouch, cylindrical, or prismatic cells.
How it works — step-by-step (charging & discharging)
Discharge (provides power to a device):
- At the cathode, sodium-hosting material releases Na⁺ ions (de-intercalation).
- Released Na⁺ ions migrate through the electrolyte and separator toward the anode.
- At the anode, Na⁺ ions are inserted into the anode host (hard carbon intercalation).
- Electrons flow through the external circuit from anode to cathode, powering the load.
Charge (stores energy):
- External power forces electrons back into the anode through the external circuit.
- Sodium ions de-insert from the anode and travel back through the electrolyte to re-insert into the cathode structure.
- The cell returns to a charged state, ready to supply energy again.
Important practical points in construction & working
- Initial irreversible capacity loss (IRC): On first charge, some Na⁺ becomes trapped forming the solid-electrolyte interphase (SEI) on hard carbon. Manufacturers use pre-sodiation steps or sacrificial salts to compensate for IRC.
- Electrode balancing: Proper cathode/anode capacity balancing is critical in sodium ion battery construction and working to avoid wasting active sodium or over-lithiation/over-sodiation of electrodes.
- SEI chemistry differs from lithium: The composition and stability of the SEI layer with Na⁺ affects cycle life and safety—researchers optimize electrolyte additives to form stable SEI.
- Temperature & ion size: Sodium ions are larger than lithium ions — this affects diffusion rates, electrode particle design, and therefore sodium ion battery construction and working choices like particle size, porosity and electrode thickness.
Performance drivers engineers tune
- Active material particle size and morphology (faster Na⁺ transport).
- Binder and conductive additive choices in electrode slurries (mechanical stability).
- Electrolyte formulation (ionic conductivity, SEI formation, temperature window).
- Electrode coating thickness and porosity (tradeoff between energy density and rate capability).
Takeaway: if you can explain the role of hard carbon anodes, layered/polyanionic cathodes, electrolyte choices, and the ion flow during charge/discharge, you’ve covered the essentials of sodium ion battery construction and working.
Sodium-Ion Battery vs Lithium-Ion Battery
Comparing sodium ion battery vs lithium ion helps readers understand where each technology fits. They share the same operating principle (ion shuttling between electrodes) but differ in materials, performance trade-offs, cost and ideal applications.
Key comparison points
Energy density
- Lithium-ion cells generally have higher energy density because lithium ions are smaller and many lithium cathode/anode chemistries pack more charge per mass/volume.
- Sodium-ion cells historically have lower energy density, but advances in cathode design and electrode engineering are narrowing the gap. In short: lithium currently leads on energy density, sodium is catching up for many stationary and low-weight-sensitive use cases.
Cycle life & calendar life
- Both chemistries can be engineered for long cycle life. Sodium-ion design choices (electrolyte additives, pre-sodiation, stable cathode chemistries) are improving cycle stability. In many cell architectures, sodium-ion cycle life is now competitive for grid and stationary applications.
Cost
- Sodium is abundant and cheap; raw-material cost for sodium salts and iron/vanadium/phosphate cathodes tends to be lower than nickel/cobalt/manganese-heavy lithium cathodes. This often makes sodium ion battery vs lithium ion a cost advantage for large-scale storage.
Safety & thermal stability
- Some sodium-ion chemistries (especially polyanionic cathodes) offer excellent thermal stability and lower fire risk than high-energy lithium chemistries. However, safety depends on full cell design and electrolyte choice—both technologies require careful thermal management.
Raw material availability & supply chain
- Lithium and some critical lithium cathode metals (cobalt, nickel) are geographically concentrated and supply-constrained. Sodium is widely available (sea salt, minerals), giving sodium ion battery vs lithium ion a clear advantage in raw material security and sustainability.
Applications where sodium is practical
- Grid-scale stationary storage, microgrids, renewable energy buffering, and low-cost EV segments (2W/3W, entry-level cars) are prime use cases for sodium-ion batteries. For high-range, weight-sensitive applications (flagship EVs, high-end consumer electronics), lithium-ion still dominates.
Clear comparison table (snapshot)
| Metric | Sodium-Ion | Lithium-Ion |
|---|---|---|
| Energy density | Lower (improving) | Higher (current leader) |
| Cycle life | Competitive (depends on chemistry) | Good to excellent |
| Cost (raw materials) | Lower (abundant Na) | Higher (Li + critical metals) |
| Safety / thermal stability | Often better with polyanionic cathodes | Depends on chemistry; some high-energy Li cells more thermally sensitive |
| Supply security | High (global sodium reserves) | Moderate (concentrated supply chain) |
| Best applications | Grid / stationary, budget EVs, backup | Mobile electronics, high-range EVs, portable devices |
Practical engineering notes
- Voltage window: Nominal cell voltages can differ depending on cathode chemistry; sodium cells may have slightly lower nominal voltages than similar lithium chemistries, which influences pack design.
- Design tradeoffs: When designing packs, engineers consider energy density versus cost, rate capability, operating temperature, and safety. This is why the question sodium ion battery vs lithium ion is not “which is overall better,” but “which is better for the application?”
- Hybrid ecosystems: Expect a future where sodium ion battery vs lithium ion is not an either/or: lithium will continue to power weight-sensitive, high-performance markets while sodium enables affordable, sustainable large-scale storage.
Takeaway: The sodium ion battery vs lithium ion debate is application-specific. Sodium brings cost, supply-security and safety advantages that make it an excellent choice for large-scale and budget-sensitive energy storage — while lithium remains preferred where highest energy density is essential.
Advantages of Sodium-Ion Batteries
The sodium ion battery is attracting worldwide attention because it solves many problems associated with lithium-ion technology. While it may not completely replace lithium batteries, it brings unique strengths that make it a promising alternative in the global energy landscape.
Raw Material Abundance and Low Cost
- Sodium is one of the most abundant elements on Earth, found in seawater and minerals worldwide.
- Unlike lithium, cobalt, and nickel (often mined under geopolitical or environmental stress), sodium sources are inexpensive and evenly distributed.
- This abundance translates into lower material costs for the sodium ion battery, making it an affordable choice for large-scale deployment.
Safer Chemistry
- The larger ionic size of sodium makes some electrode chemistries more stable under high temperature and overcharging conditions.
- Polyanionic cathodes in particular reduce oxygen release during thermal runaway, lowering the risk of fires and explosions compared to certain lithium-ion chemistries.
- This makes the sodium ion battery a safer option for stationary storage and densely packed grid-scale systems.
Ideal for Large-Scale Stationary Storage
- Grid-scale storage does not demand ultra-high energy density — instead, it needs low cost, safety, and long life.
- The sodium ion battery delivers on all three fronts, offering excellent potential for renewable energy integration (solar, wind) and backup storage.
- Because sodium is abundant and cheap, it can support mass deployment without the raw material bottlenecks lithium faces.
Environmental & Sustainability Benefits
- Lower reliance on rare or toxic metals (like cobalt) improves the overall sustainability of sodium ion battery production.
- Easy recycling and global resource availability ensure these batteries can scale up without significant environmental impact.
✅ Takeaway: The sodium ion battery offers a unique combination of affordability, safety, and scalability — making it especially well-suited for powering the renewable energy future.
Limitations and Challenges
While promising, the sodium ion battery is not a perfect solution. Like any emerging technology, it faces hurdles before achieving full commercial adoption. Comparing sodium ion battery vs lithium ion reveals both its strengths and the areas where it still lags.
Lower Energy Density
- One of the biggest challenges is the energy density gap. Lithium ions are smaller and lighter, allowing them to store more energy in a compact space.
- The sodium ion battery typically has 20–30% lower energy density compared to mainstream lithium-ion cells.
- This makes sodium less suitable for weight-sensitive applications like smartphones, laptops, or long-range EVs.
Early Stage of Commercialization
- While lithium-ion has decades of industrial experience and a vast supply chain, the sodium ion battery is still new.
- Only a few companies (e.g., CATL, Faradion, Natron Energy) are bringing sodium-ion cells to the market.
- As production scales, costs will fall, but for now lithium retains a strong lead in manufacturing maturity.
Performance in Extreme Climates
- Some sodium ion battery chemistries show lower efficiency and faster degradation in very cold or very hot climates compared to lithium-ion.
- Optimizing electrolytes and SEI stability under wide temperature ranges remains an ongoing research area.
Scaling and Industrial Adoption
- Gigafactories and infrastructure for lithium are already well-established. Shifting to sodium requires retooling and investment.
- The sodium ion battery vs lithium ion transition will likely be gradual — sodium filling niche applications (grid storage, 2W/3W EVs) before mass adoption.
✅ Takeaway: The sodium ion battery is cost-effective and sustainable, but compared to lithium-ion, it still struggles with lower energy density, limited commercialization, and performance challenges in harsh climates.
Sodium-Ion Battery Companies Leading Innovation
The global race to commercialize sodium-ion technology is gaining momentum, with several pioneering sodium ion battery companies driving research and production.
🔹 CATL (China) – The First Mass Producer
- Contemporary Amperex Technology Limited (CATL), the world’s largest lithium-ion battery maker, announced its first-generation sodium-ion battery in 2021.
- CATL is among the leading sodium ion battery companies, already building pilot lines and planning large-scale deployment for EVs and renewable energy.
🔹 Natron Energy (USA) – Grid-Focused Innovation
- Based in California, Natron Energy specializes in sodium ion battery companies targeting grid storage, data centers, and backup power.
- Their unique Prussian Blue chemistry offers fast charging, long cycle life, and excellent safety.
🔹 Faradion (UK, now Reliance-owned) – India’s Entry Point
- UK-based Faradion, acquired by Reliance New Energy in 2021, is a critical player among sodium ion battery companies.
- Through this acquisition, India now has a strong foothold in sodium-ion technology.
- Reliance aims to manufacture these batteries domestically to reduce import dependence and make affordable EVs.
🔹 HiNa Battery (China) – Academic to Industry
- HiNa Battery, a spin-off from the Chinese Academy of Sciences, is one of the earliest sodium ion battery companies to demonstrate working sodium-ion EVs.
- Their partnership with automakers and stationary storage providers highlights the growing commercial scope.
🇮🇳 The Indian Angle
- Beyond Reliance-Faradion, India’s ISRO has also experimented with sodium-ion cells for space applications.
- With Reliance backing local manufacturing, India could soon become a major hub for sodium-ion adoption.
✅ Takeaway: These sodium ion battery companies are laying the foundation for global adoption, with China leading the pack, the USA focusing on niche markets, and India emerging as a future hub.
Real-World Applications and Future Scope
The promise of the sodium ion battery extends far beyond the laboratory. Thanks to its affordability, safety, and sustainable raw materials, it has multiple real-world applications that could reshape the energy landscape.
Renewable Energy Storage
- Solar and wind energy require efficient storage to balance supply and demand.
- The sodium ion battery is ideal here because cost and scalability matter more than compact size.
- In practice, the sodium ion battery construction and working make it well-suited for grid storage due to its long cycle life and thermal stability.
Electric Vehicles (EVs)
- While high-performance EVs still rely on lithium, the sodium ion battery could power affordable two-wheelers, three-wheelers, and short-range cars.
- The sodium ion battery construction and working allow manufacturers to prioritize cost and safety over energy density, fitting perfectly with budget EV markets in India and Asia.
Backup Power & Data Centers
- Large facilities like hospitals, telecom towers, and cloud data centers need safe, long-life backup solutions.
- The sodium ion battery offers exactly that, with less fire risk and lower cost than lithium-ion.
Consumer Electronics (Future Potential)
- Although not yet optimized for smartphones or laptops, ongoing improvements in sodium ion battery construction and working may eventually bring them into mainstream gadgets.
✅ Takeaway: With diverse applications, the sodium ion battery is likely to play a complementary role alongside lithium, especially in large-scale storage and affordable EVs.
FAQ
What is a sodium-ion battery?
A sodium-ion battery is a rechargeable energy storage device that uses sodium ions instead of lithium ions to store and release electrical energy.
How does a sodium-ion battery work?
It works on the same principle as lithium-ion batteries—sodium ions move between the anode and cathode during charging and discharging, generating electricity.
Are sodium-ion batteries better than lithium-ion?
Sodium-ion batteries are cheaper and more sustainable because sodium is abundant, but they currently offer lower energy density compared to lithium-ion.
When will sodium-ion batteries be available in electric vehicles?
Some companies, like CATL and Faradion, are already piloting sodium-ion batteries. Commercial use in EVs is expected within the next 3–5 years.
Which companies are developing sodium-ion battery technology?
Leading companies include CATL (China), Faradion (UK), Natron Energy (USA), and HiNa Battery (China).
Conclusion: Will Sodium Replace Lithium?
The debate of sodium ion battery vs lithium ion often paints sodium as a “replacement” technology, but the reality is more nuanced. The sodium ion battery is unlikely to completely replace lithium-ion in the near term. Instead, it will complement it, serving different needs.
- For energy-dense applications like long-range EVs, lithium-ion will remain dominant.
- For cost-sensitive applications such as grid storage, short-range EVs, and backup power, the sodium ion battery is the better fit.
Looking ahead, the future of energy storage will likely be a hybrid ecosystem:
- Lithium-ion for high performance and compact electronics.
- Sodium ion battery for large-scale, affordable, and safer storage.
- Solid-state batteries for the next leap in efficiency and safety.
✅ Final Word: In the battle of sodium ion battery vs lithium ion, there is no single winner. The two technologies will coexist, each powering different parts of our electrified future.
⚡ Interested in more breakthroughs in energy storage? Explore these articles: