|
The Invention of battery
Imagine a world without the constant hum of your phone, the calming glow of your laptop, or the instant connection to music and information. This reality, not so long ago, highlights the transformative power of a seemingly simple invention: the battery.
The invention of battery stands as a pivotal moment in human history, marking the genesis of efficient energy storage and utilization. From the earliest electrochemical cells to the sophisticated lithium-ion batteries powering our digital era, the journey of battery technology has been shaped by numerous inventors, innovations, and significant milestones.
But the journey of the battery is far from ordinary. This exploration delves into the history of this remarkable invention, tracing its evolution from its earliest, enigmatic beginnings to the cutting-edge innovations shaping our technological future.
How Batteries Works
Batteries are ingenious devices that convert chemical energy into electrical energy through a controlled electrochemical reaction. At their core, batteries consist of two electrodes—an anode (negative) and a cathode (positive)—immersed in an electrolyte solution. When the battery is connected in a circuit, a chemical reaction occurs within it. The reaction at the anode produces electrons, releasing them into the circuit, while the cathode absorbs these electrons. This flow of electrons creates an electric current that can power devices.
The movement of electrons from the anode to the cathode generates voltage, the force that propels the electrons through the circuit. The type of materials used in the electrodes and electrolyte determines the battery’s voltage, capacity, and lifespan. Rechargeable batteries, like lithium-ion ones, can reverse this chemical reaction through an external power source, allowing them to be reused multiple times by restoring the initial state of the electrodes. Understanding this chemical dance within batteries unveils the remarkable process that fuels our modern devices.
Historical Timeline of Invention of Battery
1745: Ewald Georg von Kleist and the Leyden Jar
While not a true battery in the modern sense, Ewald Georg von Kleist’s 1745 invention of the Leyden Jar marked a crucial step towards understanding and storing electricity. This glass jar lined with metal foils acted as a capacitor, storing electrical charge for a short time. Kleist’s accidental discovery, receiving a powerful shock while experimenting, became known as the “Leyden Jar shock Leyden Jar shock” and sparked widespread scientific curiosity about electricity.
1752: Benjamin Franklin and the Myth-Busting Leyden Battery
Building upon the Leyden Jar, Benjamin Franklin’s 1752 Leyden Battery consisted of multiple Leyden Jars connected in parallel. While this increased the stored charge, it wasn’t a true battery as it couldn’t generate a continuous current. However, Franklin’s experiments with electricity, including his famous kite experiment, were instrumental in demystifying electricity and paving the way for future discoveries.
The Enigmatic Baghdad Battery: A Battery or Something Else?
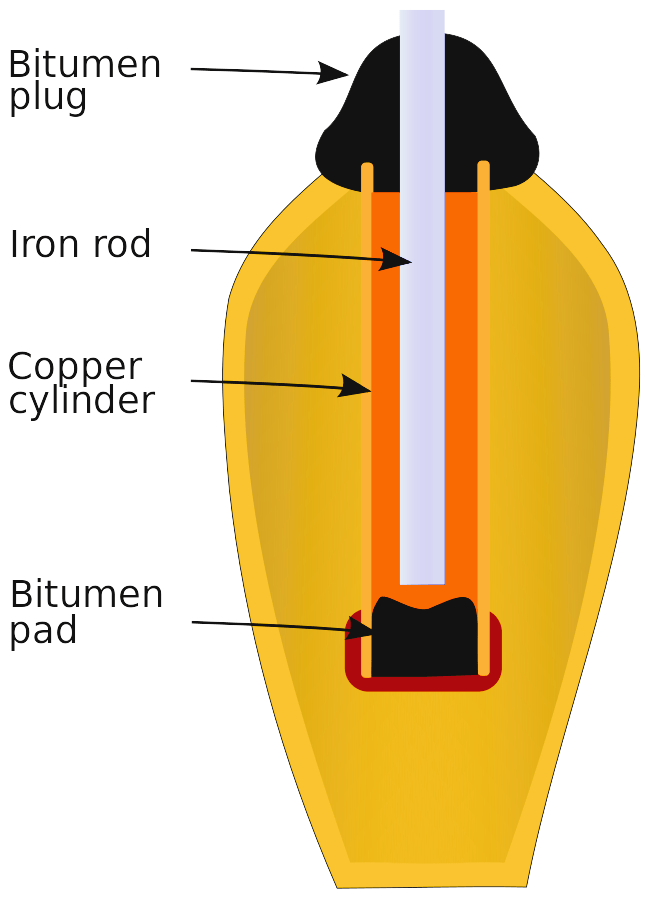
The Baghdad Battery, a clay jar containing a copper cylinder and an iron rod, has sparked debate for centuries. Dating back to the Parthian Empire (2400-2200 BC), some believe it could be an early battery. However, there’s no definitive evidence to support this claim. Studies suggest the artifact might have been used for electroplating or even for medicinal purposes. (https://en.wikipedia.org/wiki/Baghdad_Battery)
Further exploration: “https://en.wikipedia.org/wiki/Baghdad_Battery”
Beyond the Textbook: Exploring the Fringe Theories
While the historical timeline focuses on accepted scientific advancements, it’s worth mentioning some fringe theories surrounding early electricity storage. These include speculation about the use of electricity by ancient civilizations based on interpretations of hieroglyphics or depictions in artwork. However, the lack of concrete evidence makes these theories highly debatable.
A Look Ahead: The Importance of Early Discoveries
While the Leyden Jar and Leyden Battery weren’t true batteries, they were stepping stones in the history of electricity. They allowed scientists to understand the concept of electrical charge storage, paving the way for the invention of the voltaic pile, the first true chemical battery, by Alessandro Volta in 1800.
Galvani and the Beginnings of Bioelectricity
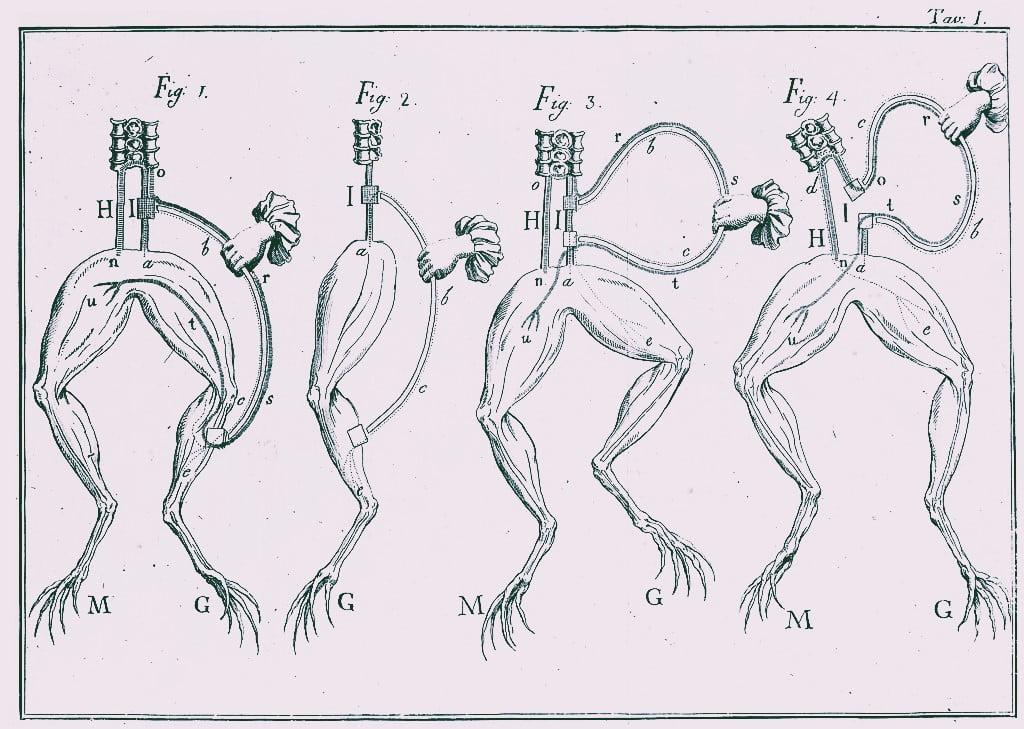
While Alessandro Volta gets the credit for the first battery (voltaic pile) in 1800, Luigi Galvani’s experiments in the late 1780s laid the groundwork. His famous experiment involving frog legs twitching when touched with different metals sparked a scientific debate. Galvani believed this was caused by “animal electricity” within the frog, while Volta disagreed, proposing a chemical reaction between the metals was generating the current.
The Importance of Galvani’s Work:
- Stimulated Debate and Research: Galvani’s work, though based on a faulty premise, ignited a crucial scientific debate about the nature of electricity. This debate directly spurred Volta’s research, ultimately leading to the invention of the voltaic pile.
- Paved the Way for Electrophysiology: Galvani’s focus on the link between electricity and muscle movement opened a new field of study: electrophysiology. This field continues to be crucial in understanding the nervous system and muscle function.
Relevance to Battery Development:
Although Galvani didn’t invent a battery, his work on “animal electricity” played a vital role in the following ways:
- Highlighted the Role of Chemistry: Galvani’s experiments, even with his mistaken interpretation, demonstrated that a chemical reaction could produce electrical effects. This concept became central to Volta’s voltaic pile, the first true battery.
- Directed Research Towards Chemical Batteries: The debate between Galvani and Volta shifted the focus from “animal electricity” to a chemical explanation. This steered research towards understanding how chemical reactions could be harnessed for electricity production, paving the way for future battery development.
Volta’s Pioneering Voltaic Pile
In 1800, Alessandro Volta, an Italian physicist, achieved a breakthrough that revolutionized the understanding and use of electricity. He invented the voltaic pile, considered the first true chemical battery capable of generating a continuous current.
Working Principle:
The voltaic pile was a simple yet ingenious design. It consisted of alternating discs of different metals, typically zinc and copper, separated by layers of brine-soaked cloth (often saltwater solution). This seemingly simple arrangement produced electricity through a fascinating interplay of chemistry and physics:
- Electrochemical Reaction: When the metals come into contact with the electrolyte (brine solution), a chemical reaction occurs at their surfaces. Zinc, being more reactive than copper, loses electrons and becomes positively charged (anode). Copper, conversely, gains electrons and becomes negatively charged (cathode).
- Electron Flow: The difference in charge creates an electrical potential between the metals. The electrolyte acts as a bridge, allowing positively charged ions (from the zinc dissolving) to flow through the solution toward the copper electrode. Meanwhile, electrons flow through an external wire connecting the zinc and copper discs, creating a continuous current.
- Stacking for Increased Power: Volta’s genius lay in stacking multiple pairs of metal discs and soaked cloth, creating a “pile.” Each additional pair added to the voltage (electrical pressure) of the overall system, providing a stronger and more sustained current.
Historical References:
- Volta’s invention was documented in a series of letters to the Royal Society of London, published between 1793 and 1800. These letters, titled “On the Electricity Excited by the Mere Contact of Conducting Substances,” detailed his experiments and the development of the voltaic pile.
- Volta’s work was met with great excitement and skepticism. His discovery sparked widespread scientific debate about the nature of electricity and the concept of “voltaic electricity” versus animal electricity (as proposed by Galvani).
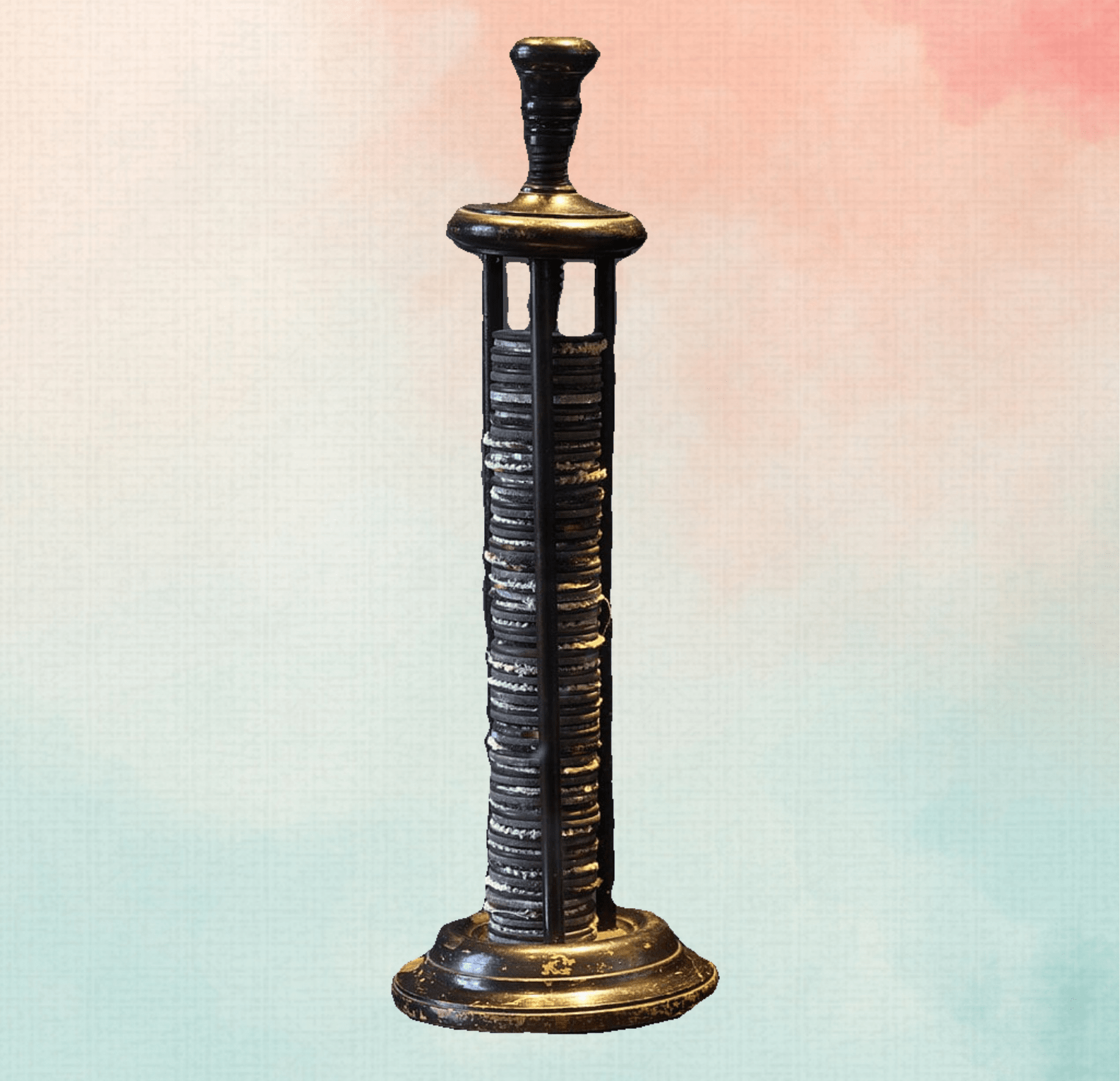
Impact and Significance:
The voltaic pile had a profound impact on the scientific understanding of electricity:
- From Static to Continuous Current: Before Volta’s invention, electricity was primarily studied through static discharges from Leyden jars. The voltaic pile provided a reliable source of continuous current, allowing for sustained experimentation and new discoveries.
- Electrolysis and Electroplating: The voltaic pile became a crucial tool for studying electrolysis, the process of separating elements from compounds using electricity. This discovery eventually led to advancements in electroplating, allowing for the deposition of metals on surfaces.
- Paving the Way for Future Batteries: The voltaic pile established the fundamental principle of using chemical reactions to generate electricity. This concept became the foundation for all future battery development, leading to the vast array of batteries we use today.
Limitations of the Voltaic Pile:
Despite its groundbreaking nature, the voltaic pile had some limitations:
- Short Lifespan: The saltwater electrolyte was corrosive and caused the zinc discs to deteriorate quickly, limiting the lifespan of the battery.
- Low Power Output: While a significant advancement, the voltaic pile produced a relatively weak current compared to modern batteries.
The Daniell Cell (1836): A Symphony of Separations for Stable Power
Working Principle:
- John Frederic Daniell’s ingenious design addressed the critical issue of internal corrosion that plagued the voltaic pile. The Daniell cell employed a two-chamber system separated by a porous earthenware partition.
-
- The positive chamber (cathode) consisted of a copper sulfate solution contained within a copper pot. As the cell discharged, copper ions (Cu²⁺) plated onto the copper electrode, preventing the detrimental hydrogen gas evolution seen in the voltaic pile.
- The negative chamber (anode) held a zinc rod immersed in sulfuric acid. During discharge, zinc atoms dissolved (oxidized) into the solution, releasing electrons that flowed through the external circuit.
- The crucial element was the porous earthenware separator. It allowed the flow of ions (essential for current generation) while preventing the copper sulfate solution from mixing directly with the sulfuric acid. This eliminated unwanted internal reactions that limited the lifespan of the voltaic pile.
Historical Significance:
- The Daniell cell offered a significant advantage – stability. Unlike the erratic current output of the voltaic pile, the Daniell cell provided a more consistent and long-lasting source of electricity. This made it ideal for applications requiring reliable current, such as:
-
- Early Telegraphy Systems: The consistent current of the Daniell cell facilitated the development of long-distance telegraphy, a vital step in communication infrastructure.
- Electrochemical Research: The stable current output of the Daniell cell proved invaluable for early electrochemists studying the relationship between electricity and chemical reactions.
While the Daniell cell is lauded for its stability, it wasn’t without limitations. The two-chamber design and the use of a fragile earthenware separator made it bulky and less portable compared to later battery inventions.
Further Exploration:
- A detailed scientific description of the Daniell cell’s construction and operation can be found in Michael Faraday’s 1836 paper, “On Voltaic Electricity“
The Grove Cell (1839): Power Up, But at a Cost
Working Principle:
- William Robert Grove’s invention built upon the Daniell cell’s design, aiming for a higher power output. The Grove cell used platinum as the positive electrode and zinc as the negative electrode, both immersed in sulfuric acid.
-
- Platinum, being a more noble metal than copper, significantly reduced the hydrogen gas evolution that limited the power output of the Daniell cell. This allowed for a higher concentration of sulfuric acid, further increasing the cell’s voltage and current.
Historical Significance:
- The Grove cell delivered on its promise of increased power. This made it suitable for applications demanding more current, such as:
-
- Early Electric Lighting: The Grove cell’s higher power output could support the rudimentary electric lamps of the era.
- Electroplating: The stronger current facilitated the electroplating process, used for applying a thin layer of metal onto another surface.
While the Grove cell offered a power boost, it came with drawbacks.
-
- Cost: Platinum is a significantly more expensive metal compared to copper used in the Daniell cell. This made the Grove cell a less economical option.
- Fumes: The concentrated sulfuric acid used in the Grove cell produced harmful fumes during operation, posing safety concerns.
The Daniell cell and the Grove cell, while distinct in their approaches, represent a crucial phase in battery development. They addressed the limitations of the voltaic pile, paving the way for future advancements in efficiency, power output, and portability.
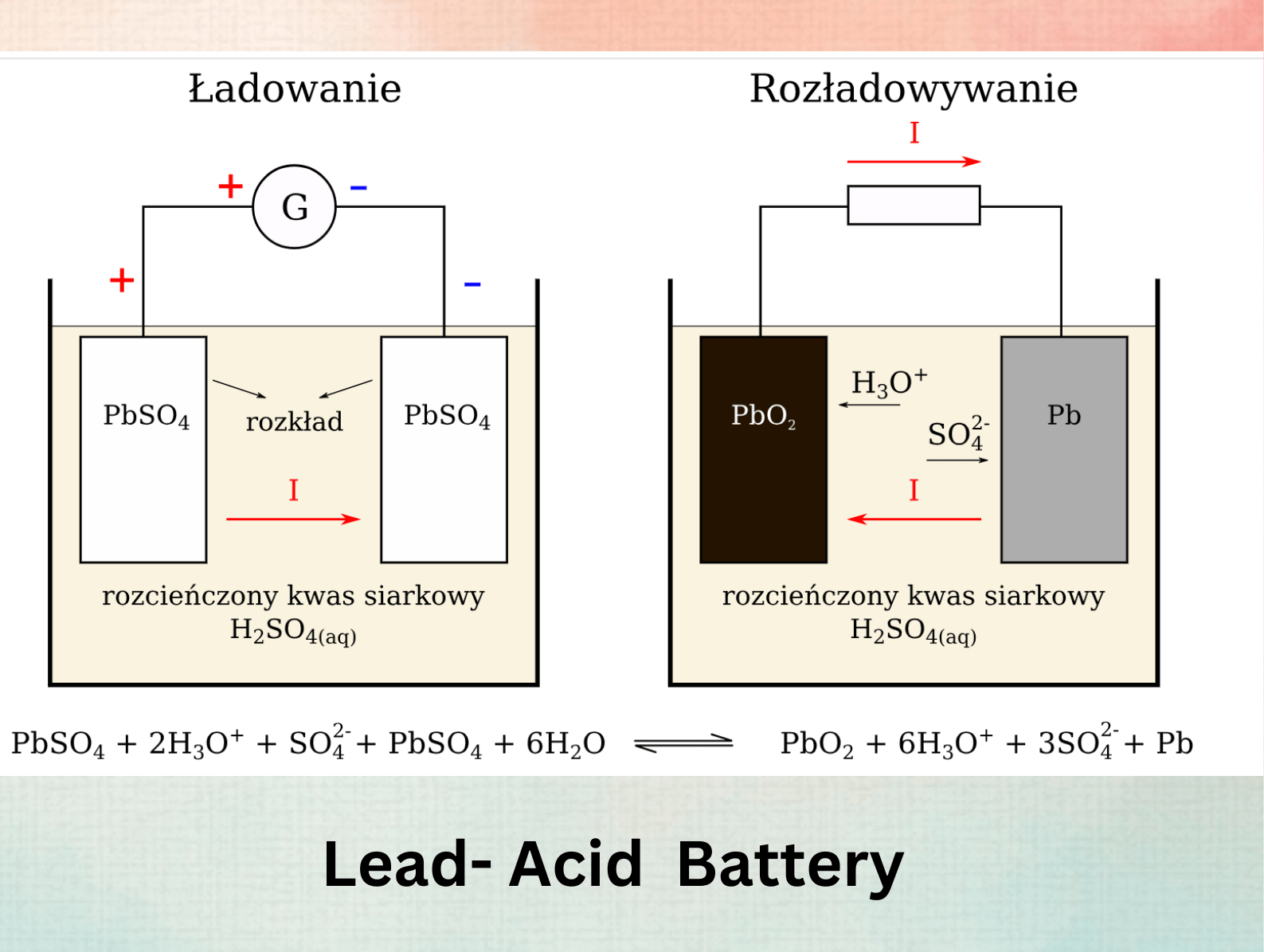
The Lead-Acid Battery (1859): A Revolutionary Recharge
Inventor: Gaston Planté, a French physicist, forever changed the game with the first practical rechargeable battery – the lead-acid battery.
Working Principle:
Unlike earlier batteries that were essentially disposable, Planté’s design offered a revolutionary concept – rechargeability. Two lead plates were immersed in sulfuric acid. During discharge, a chemical reaction between the lead plates and the acid generates electricity. The key innovation was the ability to reverse the current and recharge the battery. This involved applying a strong current, essentially “re-forming” the lead plates and restoring their ability to generate electricity.
The Early Struggles and Planté’s Persistence
Planté’s initial lead-acid battery design had limitations. The “forming” process to recharge the battery could take days, making it impractical for widespread use. However, Planté’s contribution lies in proving the concept of a rechargeable battery. His work paved the way for later advancements that addressed the charging time issue.
Historical Significance and Applications:
- Revolutionized industrial applications: The lead-acid battery provided a reliable and reusable source of electricity for powering factories, telegraph stations, and early electric vehicles.
- Paved the way for future rechargeable batteries: Planté’s invention laid the groundwork for the development of more efficient and user-friendly rechargeable batteries in the 20th century.
Further Exploration:
- Study: A detailed analysis of Planté’s work and the early lead-acid battery can be found in a seminal paper published in the “Comptes Rendus de l’Académie des Sciences (French) in 1860.
- Website: The Battery University website offers a user-friendly explanation of the lead-acid battery’s working principle
The Leclanché Cell (1866): A Portable Power Shift
Inventor: Georges Leclanché, a French chemist, addressed a key limitation of earlier battery designs – portability.
Working Principle:
The Leclanché cell, also known as the zinc-carbon cell, offered a more portable option. It employed a zinc rod as the negative electrode and a carbon rod surrounded by manganese dioxide as the positive electrode. A solution of ammonium chloride acted as the electrolyte. While not rechargeable, this design was crucial for early portable devices.
The Rise of the “Wet Cell” and its Limitations
The Leclanché cell belonged to a category known as “wet cell” batteries due to the liquid electrolyte. These wet cells offered improved performance compared to earlier dry batteries, but they were prone to leakage and required careful handling.
Historical Significance and Evolution:
- Paved the way for portable communication: The Leclanché cell became the go-to power source for portable telegraphy equipment, a vital innovation for communication infrastructure.
- Forerunner of dry cell batteries: Leclanché’s design principles formed the foundation for the later development of dry cell batteries, which replaced wet cells due to their portability and safety.
Further Exploration:
- Historical Reference: The book “The Battery: A Very Short Introduction” by Richard Pearce provides a historical perspective on the evolution of batteries, including the Leclanché cell.
- Website: The National Museum of American History website offers a closer look at the Leclanché cell and its impact on communication technology
These 19th-century breakthroughs addressed the limitations of existing batteries and opened doors for exciting new applications that would transform how we work, communicate, and eventually, interact with portable electronics.
The Nickel-Cadmium Battery (1901): A Workhorse for Portability
- Inventor: Waldemar Jungner, a Swedish inventor and engineer.
Working Principle:
- Unlike lead-acid batteries, the nickel-cadmium (NiCd) battery offered a lighter and more portable option. It employed nickel hydroxide as the positive electrode and metallic cadmium as the negative electrode, both immersed in a potassium hydroxide electrolyte. During discharge, a chemical reaction generates electricity. The NiCd battery could be recharged by reversing the current, allowing for multiple charge cycles.
While widely used in the 20th century for portable electronics like cameras and power tools, NiCd batteries faced criticism due to their environmental impact from cadmium, a toxic heavy metal. This led to regulations restricting their use and a push for cleaner alternatives.
- Study: A 1998 study published in the Journal of Power Sources compared the performance and environmental impact of NiCd batteries with other rechargeable options.
- Website: The Nickel Cadmium Battery Recycling Coalition website provides information on NiCd battery recycling and environmental considerations
The Alkaline Battery (1960s): A Champion of Shelf Life and Capacity
- Development: Several companies, including Union Carbide and Eveready, contributed to the development of the alkaline battery in the 1960s.
Working Principle:
- Building on the zinc-carbon cell design, the alkaline battery uses a similar zinc anode but with an alkaline electrolyte (potassium hydroxide or sodium hydroxide) and a manganese dioxide cathode. This combination allows for a higher energy density and longer shelf life compared to zinc-carbon batteries.
- Less Common Information: The specific formulation of the alkaline electrolyte and cathode plays a crucial role in performance. Some variations, like those containing mercury, were phased out due to environmental concerns.
- Historical Reference: A 1964 article in the Journal of The Electrochemical Society details the development and advantages of alkaline batteries:
Further Exploration:
- The American Chemical Society website offers a clear explanation of the chemistry behind alkaline batteries
The Lithium-Ion Battery (1970s): A Revolution in the Making
The late 20th century witnessed a monumental breakthrough in energy storage technology with the advent of the lithium-ion battery. Developed collaboratively by John B. Goodenough, Rachid Yazami, and Akira Yoshino in the 1970s and 1980s, this rechargeable battery introduced a transformative approach by utilizing lithium ions to move between anode and cathode materials.
- Research Intensifies: While the concept of lithium batteries existed earlier, the 1970s saw a surge in research to overcome challenges and develop a practical lithium-ion (Li-ion) battery.
Working Principle (Simplified):
Li-ion batteries don’t undergo a chemical reaction within the electrodes. Instead, lithium ions travel between the positive and negative electrodes during discharge and charge cycles. This allows for a higher energy density and lighter weight compared to previous battery technologies.

- The development of Li-ion batteries involved overcoming safety concerns related to the flammability of certain electrolytes. Ongoing research continues to refine Li-ion technology for improved safety, performance, and faster charging times.
- Website: The Britannica website provides a comprehensive overview of the history and working principle of Li-ion batteries
- Study: A 2020 study published in Nature Reviews Materials explores the future directions and challenges in Li-ion battery development
21st Century (and Beyond)
The Quest for Ultra-High Energy Density:
- The Challenge: One of the biggest hurdles in battery technology is increasing energy density – the amount of energy a battery can store per unit weight or volume. This directly impacts the range of electric vehicles, the runtime of electronics, and the feasibility of large-scale energy storage for renewable energy sources like solar and wind.
- Beyond Lithium-ion: While Lithium-ion batteries currently dominate the market, researchers are exploring alternative materials like lithium-sulfur and lithium-metal batteries. These hold the potential for significantly higher energy densities but come with challenges in terms of stability and lifespan.
- Latest Research: A 2023 study published in the journal “Nature Energy” reported the development of a novel lithium-sulfur battery with a significantly improved lifespan through the use of a graphene-based cathode structure.
The Promise of Solid-State Batteries:
- Revolutionizing Safety and Performance: Solid-state batteries replace the traditional liquid electrolyte with a solid material. This offers several advantages:
- Enhanced Safety: Solid electrolytes eliminate the risk of flammable liquid leaks, a major concern with lithium-ion batteries.
- Faster Charging and Longer Lifespan: Solid electrolytes can enable faster charging times and potentially longer battery lifespans.
- Higher Energy Density: Solid-state design can potentially lead to even higher energy densities than traditional lithium-ion batteries.
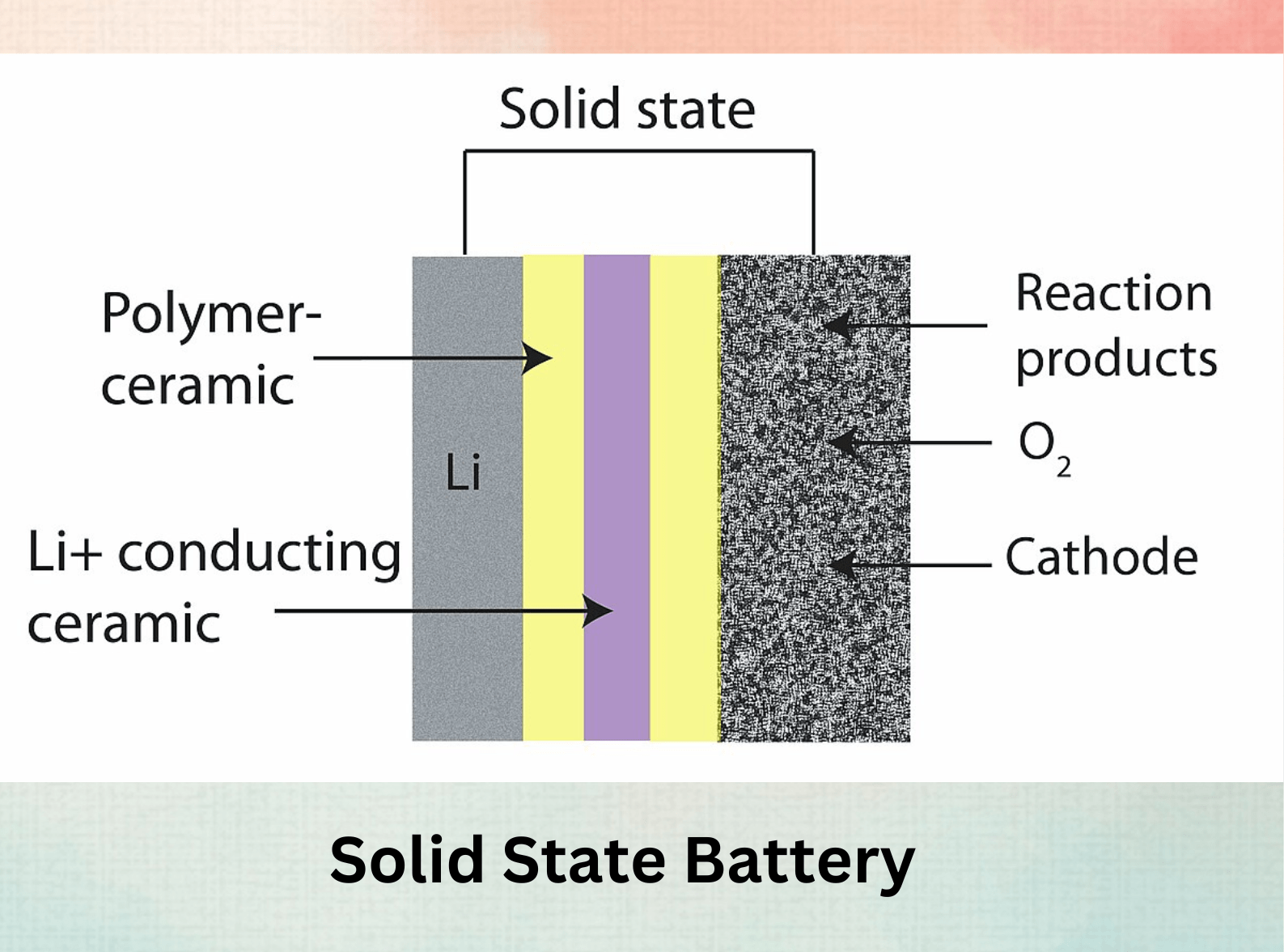
- Challenges and Progress: Developing commercially viable solid-state batteries requires overcoming challenges like finding solid electrolytes with high ionic conductivity and ensuring compatibility with electrode materials. However, significant progress is being made.
- Latest Research: A 2023 study published in the journal “Science Advances” reported the development of a new solid-state electrolyte material that shows promise for high-performance and stable solid-state batteries.
Exploring Unconventional Materials and Electrolytes:
- Beyond Lithium: While lithium remains a key player, researchers are exploring alternative battery chemistries using elements like sodium, potassium, and magnesium. These elements offer advantages like wider availability and potentially lower costs.
- Organic Materials: Some research is focused on developing organic batteries that utilize organic polymers as electrode materials. These offer potential advantages like low cost, environmental friendliness, and flexibility.
- Unique Perspectives: A recent study in “Joule” explored the potential of using wood-derived materials for sustainable and high-performance battery electrodes.
Nanotechnology and Advanced Materials: Redefining Battery Performance
The realm of nanotechnology is playing a pivotal role in pushing the boundaries of battery technology. By manipulating materials at the atomic and molecular levels, scientists are creating revolutionary innovations:
- Graphene and Beyond: Graphene, a single layer of carbon atoms arranged in a honeycomb lattice, has emerged as a superstar material. Its exceptional conductivity and large surface area make it ideal for both electrodes, potentially leading to faster charging and higher energy density. Research is also exploring other nanomaterials like carbon nanotubes and silicon nanostructures to enhance battery performance further.
- Unlocking Potential: Nanotechnology offers exciting possibilities for:
- Improved Electrode Design: Nanostructured materials can provide more surface area for reactions to occur, leading to faster charging and increased capacity.
- Enhanced Stability: Nanoengineered materials can improve the structural integrity of electrodes, extending battery lifespan.
- Safer Battery Chemistry: Nanoparticles can be used to encapsulate and stabilize reactive materials, potentially leading to safer battery operation.
Environmental Sustainability and Recycling: A Holistic Approach
As the demand for batteries surges, the environmental impact needs careful consideration. Here’s where the focus is shifting:
- Eco-Friendly Materials: Researchers are exploring alternatives to traditional battery components like cobalt and lithium, which can be environmentally damaging due to mining practices. Elements like sodium and magnesium offer promise for more sustainable battery chemistries.
- Closed-Loop Recycling: Developing efficient and cost-effective methods for recycling spent batteries is crucial. Recovering valuable materials like lithium and cobalt can minimize environmental impact and reduce reliance on virgin resources.
- Second-Life Applications: Batteries that no longer meet the demands of electric vehicles can potentially be repurposed for less demanding applications like stationary energy storage. This extends their lifespan and reduces their overall environmental footprint.
These efforts towards sustainability go hand-in-hand with advancements in materials and technology. By adopting a holistic approach, we can ensure that the future of batteries is not just powerful but also environmentally responsible.
The Future of Batteries: A Brighter Horizon
The convergence of nanotechnology, advanced materials research, and a focus on sustainability is paving the way for a transformative future of batteries. We can expect to see:
- Batteries tailored to specific applications: High-performance batteries for electric vehicles alongside safe and efficient batteries for portable electronics.
- Large-scale energy storage solutions: Enabling a wider adoption of renewable energy sources like solar and wind.
- A circular economy for batteries: Reducing reliance on virgin resources and minimizing environmental impact.

These ongoing advancements promise to revolutionize how we power our lives. From longer-range electric vehicles and more efficient portable electronics to large-scale renewable energy storage, the future of batteries is brimming with possibilities. As research continues to push the boundaries, we can expect even more groundbreaking innovations that will shape the world of tomorrow.
Conclusion: Shaping a Sustainable Energy Future
From the enigmatic Baghdad Battery to the omnipresent lithium-ion cell, the journey of the battery is a testament to human ingenuity. The quest for a reliable source of electricity has spurred centuries of scientific exploration, leading to advancements that have fundamentally reshaped our world.
Beyond Convenience: A Catalyst for Change
The impact of batteries extends far beyond powering our devices. It has:
- Revolutionized Communication: From the telegraph to the mobile phone, batteries have enabled instant and global communication, fostering collaboration and connection.
- Fueled Innovation: Portable power has driven advancements in fields like medicine, with portable diagnostic equipment, and science, with instruments for field research.
- Promised a Sustainable Future: The development of efficient and sustainable batteries is crucial for the widespread adoption of renewable energy sources, paving the way for a greener future.
Looking Ahead: A Future Powered by Progress
The story of the battery is far from over. With ongoing research in nanotechnology, new materials, and environmental sustainability, we can expect even more revolutionary breakthroughs. The future holds the promise of batteries that are:
- More Powerful: Delivering longer range for electric vehicles and extended runtime for electronics.
- Safer and More Sustainable: Minimizing environmental impact and ensuring safe operation.
- Tailored to Specific Needs: Catering to various applications from powering homes to fueling spacecraft.
The legacy of the battery is one of continuous innovation and transformative potential. As we move forward, the tireless pursuit of better battery technology will undoubtedly continue to shape the way we live, work, and connect with our world.
Check out some of my other blog posts:
- Invention Of Electricity
- War Of Currents
- Invention and History of Electric Motor
- Invention of the Light Bulb