Introduction: The Haber Process – A Double-Edged Sword
The Haber process, also known as the Haber-Bosch process, is a monumental achievement in chemical engineering that has shaped modern agriculture and industry. But what is Haber’s process, and why is it both a blessing and a challenge for our planet? At its core, the Haber process is a method of producing ammonia (NH₃) by combining nitrogen (N₂) from the atmosphere with hydrogen (H₂) in the presence of an iron-based catalyst. This reaction occurs under high temperatures (400–500°C) and pressures (150–200 atmospheres), yielding ammonia essential for synthetic fertilizers. The process can be summarized by the chemical equation:
N₂ + 3H₂ → 2NH₃
This seemingly simple reaction holds profound implications for global food security, environmental sustainability, and industrial development.
Pioneers Behind the Process
The Haber process was developed by Fritz Haber in 1909 and later scaled for industrial use by Carl Bosch in 1913. Together, these visionaries laid the foundation for modern ammonia production, enabling the mass synthesis of fertilizers. Fritz Haber’s pioneering achievement in developing a practical method for atmospheric nitrogen fixation earned him the Nobel Prize in Chemistry in 1918. Carl Bosch complemented this achievement by overcoming the technical challenges of large-scale production, such as designing reactors capable of withstanding extreme conditions. Their combined efforts revolutionized agriculture, marking a pivotal point in the history of ammonia production.

A Game-Changer in the Green Revolution
The Haber process has had an indelible impact on human history, particularly during the Green Revolution, when synthetic fertilizers became the cornerstone of modern farming. By converting atmospheric nitrogen into ammonia, the process enabled a dramatic increase in crop yields, sustaining a rapidly growing global population. Today, more than half of the nitrogen in human-consumed food originates from ammonia produced by the Haber process. Without it, feeding the current population would be nearly impossible.
The Environmental Cost of Progress
However, this revolutionary method comes with significant environmental challenges. The Haber process is one of the most energy-intensive industrial processes, consuming approximately 1% of the world’s energy supply. Its reliance on fossil fuels, particularly natural gas for hydrogen production, makes it a major contributor to greenhouse gas emissions, accounting for 1.8% of global CO₂ emissions. Additionally, the excessive use of ammonia-based fertilizers has led to nitrogen runoff, polluting water bodies and causing ecological damage, including eutrophication and dead zones in aquatic ecosystems.
Setting the Stage for Sustainable Ammonia Production
While the Haber process remains essential for modern agriculture and industrial ammonia production, its environmental toll raises critical questions about its future sustainability. As concerns about climate change and resource depletion grow, researchers are exploring innovative solutions to reduce the process’s carbon footprint and improve its efficiency. This article will delve into the limitations of the Haber process and investigate promising alternatives, such as electrochemical nitrogen reduction and advanced catalytic systems. These emerging technologies aim to redefine ammonia production for a sustainable future, ensuring food security without compromising environmental integrity.
The Invention of the Haber Process: A Double-Edged Legacy
The Haber process, pioneered in the early 20th century, represents one of the most transformative and controversial innovations in human history. It revolutionized agriculture and industry by enabling the large-scale synthesis of ammonia from atmospheric nitrogen, reshaping food production and supporting unprecedented population growth. However, Fritz Haber’s life and work reflect a dual legacy of triumph and controversy that continues to provoke reflection on scientists’ responsibilities.
The State of Agriculture Before the Haber Process
In the late 19th and early 20th centuries, global agriculture faced a severe nitrogen shortage, threatening food security for a rapidly growing population.
- Reliance on Natural Fertilizers:
Farmers depended on finite and geographically limited sources of nitrogen, such as guano deposits from Peru and nitrate mines in Chile. These resources, while effective, were rapidly depleting and unable to sustain long-term agricultural demands. - Food Scarcity and Population Pressure:
Agricultural yields were constrained with no efficient method to replenish soil nitrogen at scale. This created widespread fear of a global famine, often referred to as the “Malthusian crisis,” where food supply growth lagged behind population expansion.
The critical need for an alternative nitrogen source set the stage for one of the most significant scientific breakthroughs of the 20th century.
The Inventor: Fritz Haber
Fritz Haber, a German chemist, developed the foundational process for synthesizing ammonia in 1909. His work marked a pivotal moment in industrial chemistry and agriculture.
- The Breakthrough:
Haber converted atmospheric nitrogen (N₂) into ammonia (NH₃) using a high-pressure system, elevated temperatures, and an iron-based catalyst. This solved the monumental challenge of breaking the nitrogen molecule’s strong triple bond, which had previously rendered atmospheric nitrogen inert and inaccessible to plants. - Collaboration with Carl Bosch:
Scaling this laboratory discovery to industrial levels was achieved through collaboration with Carl Bosch, an engineer at BASF. Bosch adapted Haber’s process for mass production by designing robust high-pressure reactors and other industrial innovations, resulting in the Haber-Bosch process. - Recognition and Legacy:
In 1918, Fritz Haber was awarded the Nobel Prize in Chemistry for this achievement, as it was acknowledged as a solution to global food shortages. The synergy between Haber’s scientific ingenuity and Bosch’s engineering expertise created a cornerstone of modern agriculture.

How the Haber Process Supported a Growing Population
The Haber process dramatically increased food production, playing an indispensable role in global agricultural development.
- Agricultural Revolution:
Synthetic ammonia fertilizers provided a steady nitrogen source, significantly boosting crop yields and addressing chronic food shortages. This enabled the agricultural output required to sustain an ever-growing population. - Averting Famines:
Experts estimate that approximately 50% of the world’s current population is sustained by crops grown with fertilizers derived from the Haber process. This innovation underpinned the Green Revolution, a period marked by rapid advancements in agricultural techniques and productivity during the mid-20th century.
The Controversial Legacy of Fritz Haber
Fritz Haber’s contributions extended beyond agriculture, with his involvement in wartime chemical weaponry casting a shadow over his legacy.
- Contributions to War:
During World War I, Haber applied his expertise to develop and deploy chemical weapons, including chlorine gas, in direct violation of the Hague Convention. These efforts earned him the moniker “Father of Chemical Warfare,” a title that drew widespread condemnation. - Personal Tragedy:
Haber’s wife, Clara Immerwahr, a chemist herself, opposed his involvement in chemical warfare. Her despair over his work reportedly led to her tragic suicide shortly after Haber’s first deployment of chlorine gas. This personal loss deeply affected Haber but did not deter him from his wartime endeavors. - Dual Legacy:
Haber is celebrated for his groundbreaking contribution to feeding billions but simultaneously criticized for his role in advancing chemical weapons, highlighting the complex interplay between scientific progress and ethical accountability.
The Ethical Paradox
The invention of the Haber process underscores a profound ethical dilemma that continues to resonate in scientific discussions.
- Positive Impacts:
The Haber process has saved billions of lives by mitigating famine and enabling the agricultural output necessary for rapid population growth. - Negative Consequences:
- Environmental Costs: Excess nitrogen runoff from fertilizers contributes to soil degradation, waterway pollution, and greenhouse gas emissions, making it a significant driver of environmental challenges.
- Wartime Legacy: The chemical expertise that enabled the Haber process was also weaponized, leading to devastating consequences during World War I.
- Debates on Scientific Responsibility:
The dual nature of Haber’s contributions raises critical questions about the responsibilities of scientists in anticipating and mitigating the unintended consequences of their innovations.
Fritz Haber’s story exemplifies both the immense potential and the ethical complexity of scientific innovation. While the Haber process sustains life on Earth, it also serves as a cautionary tale about the costs of progress, highlighting the importance of integrating ethical considerations into the pursuit of knowledge.
What is Haber’s Process?: The Haber-Bosch Process in Detail
The Haber-Bosch process mechanism is a cornerstone of industrial chemistry, enabling the large-scale synthesis of ammonia, a critical compound for fertilizers and other chemical applications. This section delves into the intricate mechanics of this process, emphasizing the importance of the catalyst of Haber process, the extreme operating conditions, and the principles that govern its efficiency.
The Balanced Chemical Equation and Stoichiometry
At its core, the Haber-Bosch process converts nitrogen gas (N₂) from the atmosphere and hydrogen gas (H₂) derived from natural gas or other sources into ammonia (NH₃). The process is expressed through the balanced chemical equation:
N₂ + 3H₂ → 2NH₃
This reaction is exothermic, releasing energy as heat. Each mole of nitrogen reacts with three moles of hydrogen to yield two moles of ammonia. The stoichiometry is critical for maintaining the efficiency of the process and ensuring minimal wastage of reactants.
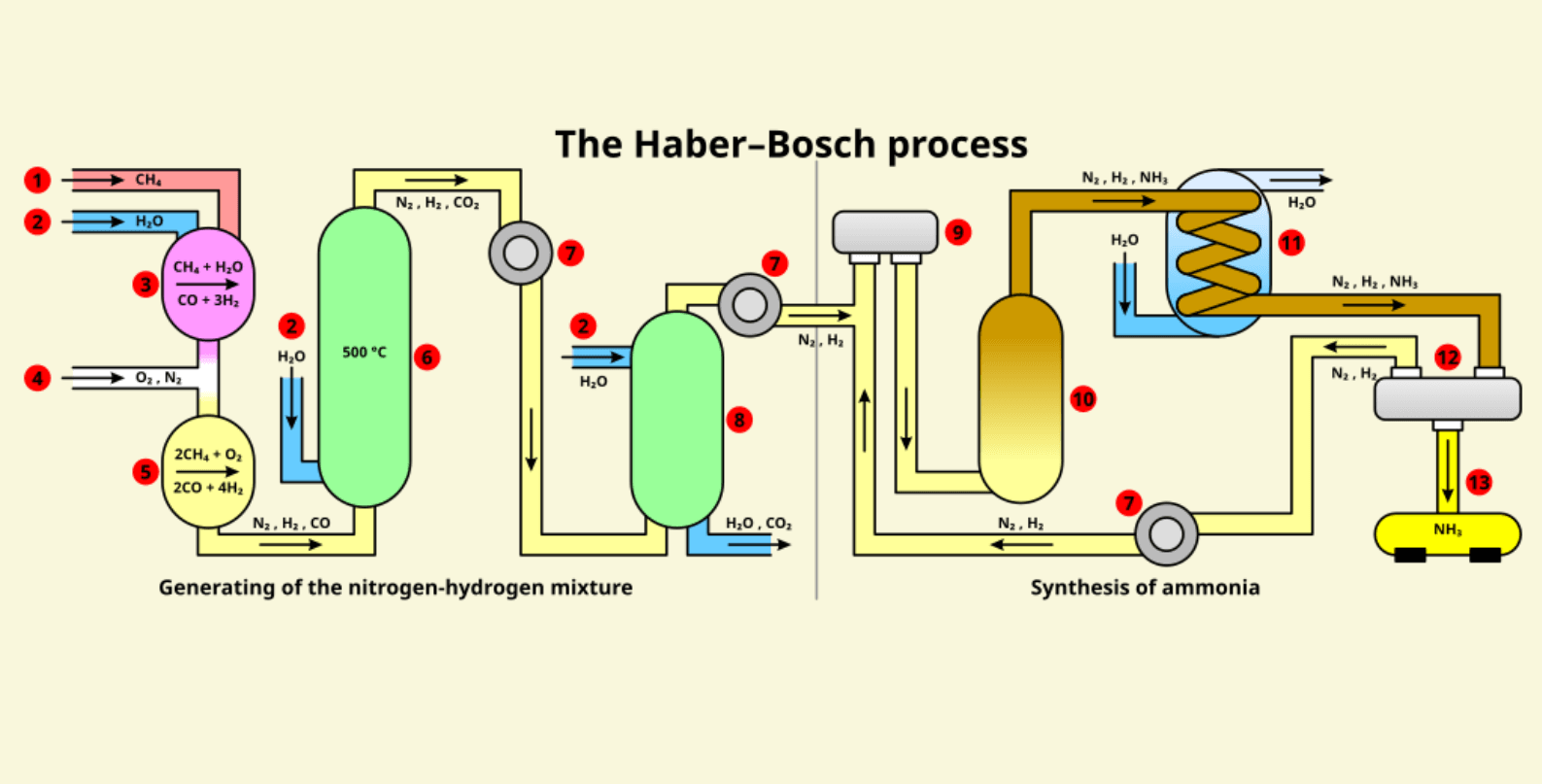
Industrial Setup: Engineering the Haber-Bosch Process
The Haber process conditions require a highly specialized industrial setup to achieve and sustain the extreme temperatures and pressures necessary for the reaction. The key components of the setup include:
- Reactors: Large steel vessels capable of withstanding high pressures (150–250 atmospheres) and temperatures (400–500°C). These reactors house the catalyst beds where the reaction takes place.
- Compressors: Used to pressurize the reactant gases, ensuring they reach the optimal pressure for ammonia synthesis.
- Heat Exchangers: Help recover and reuse the heat generated during the reaction, improving energy efficiency.
- Gas Recycling Systems: Unreacted nitrogen and hydrogen are recycled back into the reactor to maximize yield.
The Catalyst of Haber Process: A Molecular Perspective
The iron catalyst, often magnetite (Fe₃O₄), lies at the heart of the Haber-Bosch process mechanism. It plays a crucial role in facilitating the reaction by lowering the activation energy required for the conversion of nitrogen and hydrogen into ammonia.
- Why Iron Is Used
- Iron catalyst is abundant, cost-effective, and highly effective for the dissociation of molecular nitrogen (N₂), which has one of the strongest triple bonds in chemistry.
- Promoters like potassium and aluminum oxides are added to enhance its efficiency and longevity.
- How the Catalyst Works at a Molecular Level
- Adsorption: Nitrogen (N₂) and hydrogen (H₂) molecules adsorb onto the catalyst’s surface.
- Dissociation: The strong N≡N bond is broken, and the nitrogen atoms become reactive intermediates.
- Reaction: The adsorbed nitrogen and hydrogen atoms combine to form ammonia (NH₃), which desorbs from the surface.
- Limitations and Ongoing Research
- The current iron catalyst requires high temperatures and pressures, which increase energy consumption.
- Research is ongoing to develop alternative catalysts, such as ruthenium-based or electrocatalysts, that could work efficiently under milder conditions, reducing the environmental impact.
The Importance of Extreme Conditions in the Haber Process
The Haber process conditions—high temperature (400–500°C) and pressure (150–250 atm)—are essential for achieving a viable reaction rate and yield.
- High Temperature
- High temperatures increase the reaction rate by providing the energy required to overcome the activation barrier.
- However, because the reaction is exothermic, higher temperatures reduce the equilibrium yield of ammonia.
- High Pressure
- Increasing pressure shifts the equilibrium toward the formation of ammonia, as predicted by Le Chatelier’s principle (fewer gas molecules are produced).
- The trade-off is the substantial energy required to maintain these pressures, which contributes to the overall carbon footprint of the process.
The Environmental Footprint: Impact of the Haber Process on the Planet
The Haber process environmental impact is a complex issue, balancing the immense benefits of increased agricultural productivity with significant harm to the planet. While the process revolutionized food production, enabling us to sustain a growing global population, its environmental footprint has become an urgent concern. A deeper exploration of the environmental effects of ammonia production reveals its far-reaching consequences and underscores the need for sustainable alternatives.
Energy Consumption and Carbon Emissions
The Haber process is one of the most energy-intensive industrial operations in the world. Producing ammonia at the scale required for global agriculture demands tremendous amounts of energy and contributes heavily to greenhouse gas emissions.
- Global Energy Demand
- The Haber-Bosch process consumes approximately 1-2% of the world’s total energy supply, making it one of the largest single contributors to industrial energy usage.
- Most of this energy comes from fossil fuels, particularly natural gas, used to generate hydrogen through steam methane reforming.
- Carbon Emissions
- The production of ammonia generates around 450 million metric tons of CO₂ annually, accounting for roughly 1.8% of global emissions.
- If the ammonia industry were a country, its emissions would rank among the top 10 globally.
- Hydrogen production alone contributes 70–90% of the carbon footprint of the process, underscoring the need for decarbonization strategies, such as using green hydrogen.
The sheer scale of the carbon footprint tied to industrial ammonia synthesis highlights the necessity for cleaner energy sources and innovative production technologies.
Disruption of the Nitrogen Cycle
The Haber process produces vast quantities of reactive nitrogen (Nr), disrupting the natural nitrogen cycle that has operated for billions of years. Reactive nitrogen includes compounds like ammonia (NH₃), nitrates (NO₃⁻), and nitrous oxide (N₂O), which remain in the environment, causing cascading effects.
- Nitrogen Cascade
- Once introduced into the environment, reactive nitrogen moves through air, water, and soil, triggering a series of environmental issues.
- Unlike atmospheric nitrogen (N₂), which is inert and harmless, reactive nitrogen persists, amplifying its negative impacts over time.
The disruption of this delicate cycle has far-reaching consequences for ecosystems, water quality, and climate stability.
Environmental Consequences of Excess Reactive Nitrogen
Eutrophication and Dead Zones
One of the most visible consequences of nitrogen runoff is eutrophication, a process where excess nutrients lead to algal blooms in water bodies.
- How It Happens:
- Fertilizers containing ammonia and nitrates wash off from agricultural fields into rivers, lakes, and oceans.
- The overabundance of nutrients fuels rapid algal growth, which depletes oxygen levels as algae decay, creating dead zones devoid of marine life.
- Real-World Impact:
- The Gulf of Mexico dead zone, caused by nitrogen runoff from the Mississippi River basin, is one of the largest in the world, spanning over 6,000 square miles annually.
Greenhouse Gas Emissions (N₂O)
Nitrous oxide (N₂O), released during the breakdown of fertilizers, is a potent greenhouse gas.
- Global Warming Potential (GWP):
- N₂O is approximately 300 times more effective at trapping heat than CO₂.
- It contributes to about 6% of total global greenhouse gas emissions.
- Atmospheric Longevity:
- N₂O remains in the atmosphere for over 100 years, making it a long-term climate threat.
Soil Acidification
Excess nitrogen also alters soil chemistry, leading to soil acidification, which reduces its fertility and long-term agricultural productivity.
- Acidic soils lose essential nutrients like calcium and magnesium.
- Acidification affects microbial communities crucial for soil health, impairing crop growth.
Loss of Biodiversity
The widespread use of nitrogen-based fertilizers negatively affects ecosystems.
- Sensitive species are often outcompeted by nitrogen-tolerant plants, reducing biodiversity in affected regions.
- Acidified water bodies harm aquatic species, disrupting food chains and ecological balance.
Quantifying the Environmental Cost of Fertilizers
While the Haber process has been pivotal in supporting nearly 50% of the global population, its environmental costs are staggering.
- An estimated 80% of the nitrogen in fertilizers applied to fields is lost to the environment rather than being absorbed by crops.
- Excess nitrogen runoff contributes to global damages estimated at over $200 billion annually, including impacts on water quality, public health, and ecosystems.
Toward Sustainable Agriculture
Mitigating the environmental effects of ammonia production requires systemic changes in how fertilizers are produced and applied:
- Precision Agriculture: Technologies like GPS-guided equipment and soil sensors can help farmers apply fertilizers more efficiently, reducing runoff.
- Alternative Fertilizers: Research into organic and slow-release fertilizers offers potential to minimize nitrogen loss.
- Biological Nitrogen Fixation: Harnessing nitrogen-fixing microbes could reduce dependence on synthetic fertilizers.
- Circular Nitrogen Economy: Recycling nitrogen from waste streams is another promising avenue for sustainable agriculture.
By addressing the environmental cost of fertilizers, we can move toward a future where food security does not come at the expense of planetary health.
Alternatives to the Haber Process: Towards Sustainable Ammonia Synthesis
As the environmental costs of the Haber process become increasingly apparent, the search for alternatives to the Haber process has intensified. The goal is to develop methods for sustainable ammonia production that minimize energy consumption, reduce greenhouse gas emissions, and integrate seamlessly with renewable energy systems. These innovations in green ammonia synthesis represent the future of nitrogen-based fertilizers and sustainable industrial practices.
The Need for Sustainable Ammonia Production
The Haber-Bosch process, though revolutionary, is a relic of fossil fuel dependence, consuming vast amounts of natural gas and releasing significant CO₂ emissions. To meet global ammonia demand—currently exceeding 180 million metric tons annually—without exacerbating climate change, sustainable alternatives are imperative.
Green ammonia, produced using renewable energy sources, aligns with global efforts to transition toward decarbonized industries and sustainable agriculture practices. This section explores promising approaches to ammonia synthesis that could replace or supplement the Haber process.
Electrochemical Nitrogen Reduction (eNRR)
Electrochemical nitrogen reduction (eNRR) is a cutting-edge approach that uses electricity to convert nitrogen (N₂) directly into ammonia (NH₃) under ambient conditions.
- The Mechanism
- The eNRR process involves nitrogen molecules reacting with protons and electrons at an electrocatalyst surface within an electrochemical cell.
- Unlike the Haber process, which operates under extreme high temperature and high pressure, eNRR operates at room temperature and atmospheric pressure.
- Potential for Renewable Energy Integration
- Coupling eNRR systems with renewable energy sources such as solar or wind power could yield green ammonia, free from fossil fuel emissions.
- The decentralized nature of eNRR systems could enable ammonia production in remote areas without access to traditional industrial infrastructure.
- Challenges
- Low Efficiency: Current systems exhibit low Faradaic efficiency, meaning most of the input energy is lost rather than converted into ammonia.
- Catalyst Limitations: While various materials, including transition metals, metal oxides, and molecular complexes, are being explored as electrocatalysts, none have demonstrated the stability and activity needed for large-scale deployment.
- Scalability: Achieving industrial-scale production while maintaining high efficiency remains a significant hurdle.
Despite these challenges, ongoing research into advanced catalysts, improved cell designs, and process optimization holds promise for commercializing eNRR.
Biological Nitrogen Fixation
Nature has been synthesizing ammonia for millions of years through biological nitrogen fixation, a process carried out by microorganisms such as rhizobia and cyanobacteria using nitrogenase enzymes.
- The Natural Process
- Nitrogenase enzymes convert inert atmospheric nitrogen (N₂) into ammonia, which plants can readily absorb.
- This process occurs under mild conditions, making it an energy-efficient alternative to the Haber-Bosch process.
- Industrial Mimicry
- Scientists are exploring ways to harness or mimic biological nitrogen fixation in controlled environments.
- Genetic engineering efforts aim to transfer nitrogen-fixing capabilities to non-leguminous crops, reducing dependence on synthetic fertilizers.
- Challenges
- Efficiency: The nitrogenase enzyme operates slowly compared to industrial catalysts, limiting its potential for high-yield production.
- Oxygen Sensitivity: Nitrogenase enzymes are inactivated by oxygen, complicating efforts to replicate the process in industrial systems.
While biological nitrogen fixation is not yet ready for industrial-scale application, its potential for integration into sustainable agriculture makes it a critical area of research.
Other Emerging Alternatives
Plasma-Based Nitrogen Fixation
Plasma nitrogen fixation employs high-energy plasma to activate nitrogen molecules, enabling their reaction with hydrogen or oxygen to form ammonia or nitrogen oxides.
- Advantages:
- Operates under ambient conditions and can be powered by renewable electricity.
- Compact systems make it suitable for decentralized production.
- Challenges:
- High energy consumption remains a barrier to widespread adoption.
Thermochemical Processes with Alternative Catalysts
Research into novel catalysts aims to lower the energy and pressure requirements of ammonia synthesis.
- Examples include perovskite oxides and single-atom catalysts, which show promise in operating at temperatures below 300°C.
- While promising, these processes require further development to achieve commercial viability.
Hybrid Approaches
Combining multiple methods, such as using plasma to activate nitrogen followed by an electrochemical reduction step, offers a hybrid solution.
- These approaches could maximize efficiency by leveraging the strengths of different technologies.
The Path Forward
To transition from traditional ammonia synthesis to sustainable ammonia production, significant investments in research, infrastructure, and policy are required.
- Policy Support: Governments must incentivize the adoption of green ammonia technologies through subsidies, carbon pricing, and funding for research.
- Industry Collaboration: Partnerships between academia, startups, and industry leaders will accelerate innovation and deployment.
- Public Awareness: Educating farmers and consumers about the benefits of green fertilizers will drive demand and adoption.
By embracing alternatives to the Haber process, we can reduce the environmental cost of fertilizers, advance the goal of sustainable agriculture, and safeguard the planet for future generations.
The Future of Ammonia Production: A Sustainable Vision
The future of ammonia production lies in embracing green technology and advancing the transition to sustainable fertilizers. As ammonia is critical not only for global food security but also for emerging applications in energy and industry, shifting toward sustainable production methods is imperative. This vision involves the integration of renewable energy, breakthroughs in catalyst design, supportive policies, and innovative applications.
Integrating Renewable Energy Sources
Renewable energy sources such as solar, wind, and hydroelectric power are pivotal to achieving a sustainable ammonia production model. These energy sources can provide the electricity required for processes like electrochemical nitrogen reduction (eNRR) and plasma nitrogen fixation, eliminating the dependence on fossil fuels.
- Renewable Energy Synergy:
- By coupling ammonia production systems with renewable energy, the concept of green ammonia becomes feasible.
- Excess electricity from renewable sources can be stored in the form of ammonia, providing a solution to the intermittency issues of renewable energy.
- Global Potential:
- Regions with abundant renewable energy resources, such as deserts with high solar radiation or windy coastal areas, could become hubs for green ammonia production.
- This shift would democratize ammonia production, reducing reliance on centralized, fossil-fuel-based systems.
Advancing Research and Development
Continued innovation is necessary to overcome the technical and economic challenges associated with alternative ammonia synthesis methods. Key areas of focus include:
- Catalyst Design:
- Developing catalysts with higher efficiency and durability for electrochemical and thermochemical nitrogen fixation is crucial.
- Recent advances in single-atom catalysts and perovskite oxides show promise but require further optimization.
- Process Optimization:
- Enhancing reaction rates and scaling up alternative production methods will make them competitive with the Haber process.
- Reactor Engineering:
- Designing reactors that operate under ambient conditions while maintaining high throughput is a critical challenge for commercialization.
Investing in these areas will accelerate the adoption of sustainable ammonia production technologies.
Policy Implications and Incentives
Government policies and incentives will play a critical role in promoting the transition to sustainable ammonia production.
- Carbon Pricing and Emission Regulations:
- Implementing carbon taxes or emissions trading systems can make traditional ammonia production less economically attractive, encouraging green alternatives.
- Subsidies for Green Technologies:
- Providing financial support for renewable energy-powered ammonia plants and R&D initiatives can stimulate innovation and adoption.
- Global Cooperation:
- International agreements and collaboration can ensure equitable access to sustainable ammonia technologies, particularly for developing countries.
Policy frameworks that align economic incentives with environmental goals will be pivotal in driving change.
The Ammonia Economy: Beyond Fertilizers
Ammonia’s role is expanding beyond its traditional use in agriculture, offering promising applications in energy storage and transportation.
- Energy Storage:
- Ammonia can store renewable energy in a compact, transportable form. When needed, it can be decomposed to release hydrogen or used directly in ammonia fuel cells.
- Transportation Fuel:
- Ammonia-powered engines and fuel cells are being explored as alternatives to fossil fuels, especially in the shipping industry.
- Hydrogen Carrier:
- As a dense hydrogen carrier, ammonia can facilitate the development of a global hydrogen economy.
The emergence of an ammonia economy could position ammonia as a cornerstone of sustainable energy systems.
A Sustainable Future for Ammonia
The transition to sustainable ammonia production is not without challenges, but the potential benefits for sustainable agriculture, energy, and the environment are immense. Achieving this vision requires a collective effort involving technological innovation, supportive policies, and global cooperation. With the right investments, the future of ammonia production can align with the broader goals of a sustainable and equitable world.
Conclusion
Ammonia remains indispensable to modern society, playing a critical role in agriculture and emerging energy applications. However, the Haber process, while revolutionary, comes with significant environmental costs, including high energy consumption, greenhouse gas emissions, and disruption of the nitrogen cycle.
Innovative methods such as electrochemical nitrogen reduction, biological nitrogen fixation, and plasma-based nitrogen fixation offer promising alternatives, paving the way for a sustainable future. These approaches, combined with the integration of renewable energy and advanced catalyst technologies, hold the potential to revolutionize ammonia production.
To realize this future, continued research, development, and investment are essential. Equally important are policy incentives that support green ammonia technologies and foster global collaboration.
By embracing green technology, we can mitigate the environmental impact of ammonia production, ensure food security for future generations, and unlock ammonia’s potential in a sustainable energy economy. The journey toward a more sustainable world begins with reimagining how we produce this vital compound.
Explore below interesting articles:
- Satellite Farming
- The Role of Bioengineering in Modern Agriculture
- Robotics in Agriculture
- Innovative Vertical Farming Technologies Explored
- Drone-Assisted Pollination in Modern Agriculture