Introduction to Cryo-Electron Microscopy
Cryo-electron microscopy (cryo-EM) has emerged as a transformative tool in the field of structural biology, allowing scientists to visualize biological molecules in near-native states. Unlike traditional microscopy methods, which can alter the structure of the sample through staining or crystallization, cryo-EM uses rapid freezing to preserve the biological sample’s integrity. This technique enables the study of molecules at resolutions that were previously unattainable, contributing to significant advancements in areas such as molecular biology, structural biology, and drug discovery.

What is Cryo-Electron Microscopy?
Cryo-EM is a type of electron microscopy where samples are frozen to cryogenic temperatures and observed under an electron beam. The key advantage of cryo-EM is its ability to image biological samples without damaging them or requiring elaborate sample preparation. Cryo-EM achieves this by freezing samples in thin layers of ice, ensuring that their natural structure is maintained.
In comparison to other imaging techniques like X-ray crystallography or nuclear magnetic resonance (NMR), cryo-EM is particularly effective at capturing large, complex molecules that may not easily form crystals. Its application ranges from visualizing small proteins to large viruses, making it a game-changer in biological and medical research.
The History and Nobel Prize: Why Cryo-EM Deserved It
The recognition of cryo-EM as a revolutionary technique was cemented when Jacques Dubochet, Joachim Frank, and Richard Henderson were awarded the Cyro electron microscopy Nobel Prize in Chemistry in 2017 for their contributions to its development. The award highlighted the technology’s immense impact on biological sciences, particularly its ability to visualize biological molecules in atomic detail without the need for crystallization.
A Revolutionary Discovery
Before cryo-EM, methods like X-ray crystallography were limited in their capacity to observe certain biological molecules, especially those that did not crystallize well. Cryo-EM changed the game by allowing scientists to bypass these limitations, providing detailed images of biomolecules, viruses, and cellular structures in their natural environment.
Impact on Science
Since the development of cryo-EM, its application has led to several groundbreaking discoveries in biology and medicine. Notably, researchers have used cryo-EM to study viruses such as HIV, Zika, and SARS-CoV-2, providing insights that are crucial for vaccine and drug development. Moreover, cryo-EM has enabled the visualization of complex proteins like G-protein coupled receptors (GPCRs), which play a pivotal role in cell signaling and are major drug targets.
Notable Examples
One of the most significant achievements made possible by cryo-EM was the resolution of the Zika virus structure be, which was crucial in understanding its mechanism of infection and aiding in vaccine development. Similarly, cryo-EM has been instrumental in studying the molecular structure of HIV, helping scientists develop more effective antiviral treatments.
What is Cryo-Electron Microscopy and How Does It Work?
Cryo-electron microscopy is a specialized form of electron microscopy that allows researchers to observe the structure of biological molecules at atomic resolution. The technique leverages extremely low temperatures to freeze biological samples, preserving their native structure for high-resolution imaging.
How Does Cryo-EM Work?
The cryo-EM process starts with sample preparation, where the biological sample is suspended in a thin layer of water and rapidly frozen in liquid ethane to form a vitreous ice layer. This prevents the formation of ice crystals, which could distort the image. The frozen sample is then transferred to the electron microscope, where it is imaged under low-dose electron beams to prevent radiation damage.
The microscope captures numerous two-dimensional (2D) images of the sample from different angles, which are then computationally reconstructed into a three-dimensional (3D) model. This 3D model provides detailed information about the structure of the molecule, allowing researchers to study its function and interactions.
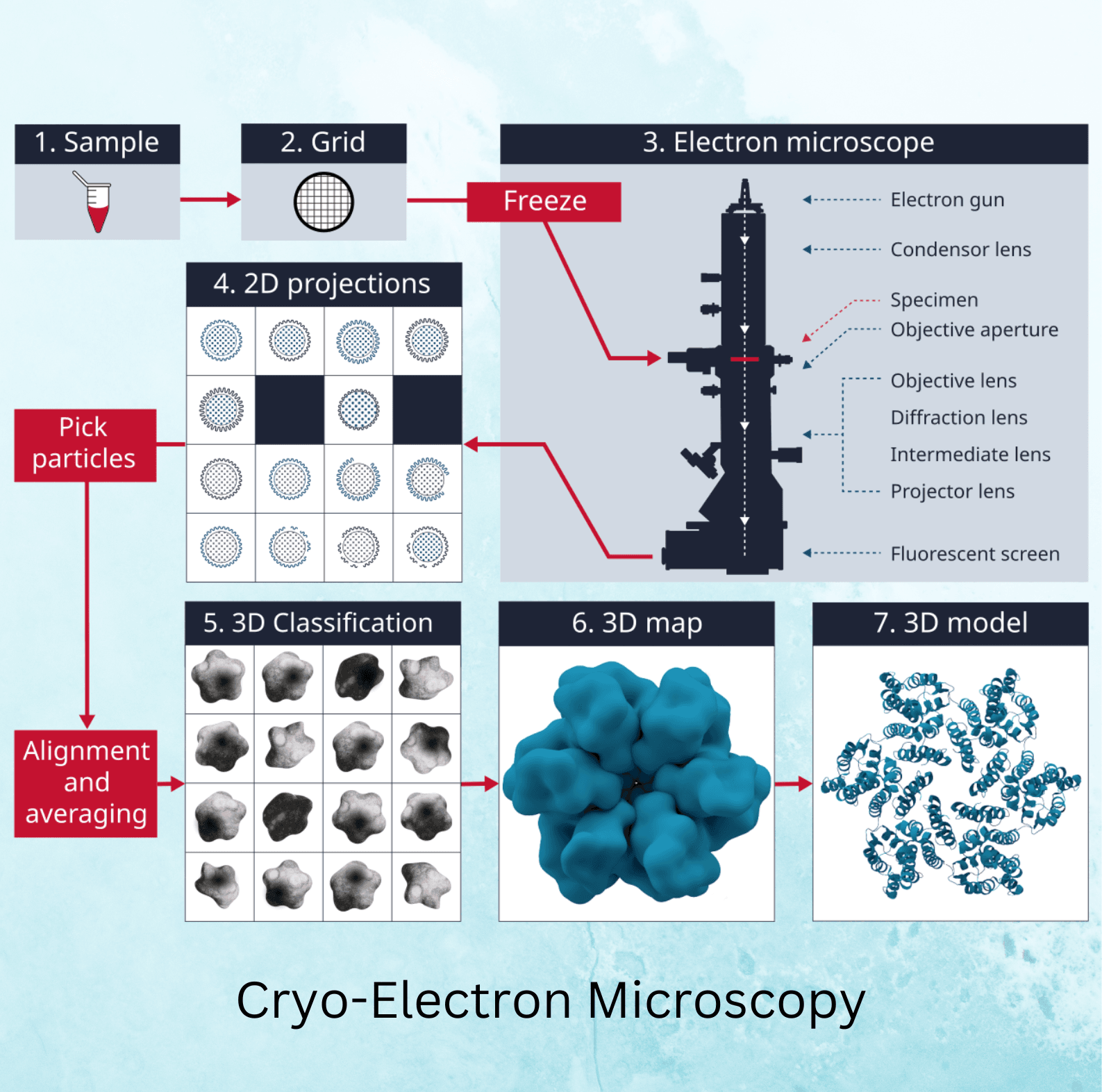
Cryo-EM vs. Other Methods
Cryo-EM offers several advantages over traditional methods like X-ray crystallography and NMR spectroscopy. While X-ray crystallography requires the sample to form a crystal, cryo-EM can image samples in their natural state without crystallization. Additionally, cryo-EM is well-suited for studying large and dynamic molecules that are difficult to analyze using other methods.
Cryo-Electron Microscopy Sample Preparation: The Most Critical Step
Importance of Sample Prep:
Sample preparation is the backbone of successful cryo-electron microscopy (cryo-EM), as the quality of your results depends heavily on how well the sample is preserved during freezing. The process must maintain the sample in its near-native state without ice crystal formation, which can distort the structural details captured by cryo-electron microscopy. By freezing the sample at ultra-low temperatures, scientists ensure that molecules remain fixed in their natural configurations, crucial for reliable imaging results. Cryo-electron microscopy sample preparation is often seen as the most challenging step because improper freezing can damage or alter biological structures, rendering the data useless.
Methods of Preparation:
The most common method used in cryo-electron microscopy sample preparation is plunge freezing, where the sample is quickly immersed in liquid ethane to vitrify the water. This technique ensures that the sample freezes so rapidly that ice crystals cannot form, preserving the biological material in its original state. Another vital method is vitrification, a process where samples are flash-frozen into a glass-like solid without crystallization. These methods are crucial in cryo-electron microscopy to achieve the high resolution needed for imaging.
Common Pitfalls:
While cryo-electron microscopy sample preparation is a powerful technique, it is also prone to errors. One of the most common challenges is contamination—tiny particles or contaminants on the sample surface can obscure important molecular details during imaging. Another pitfall is mechanical damage caused by mishandling during freezing. Over-freezing or under-freezing can introduce artifacts, making accurate interpretation of the sample difficult. Mastery of cryo-electron microscopy sample handling is key to overcoming these obstacles and obtaining clear, high-resolution images.
Real-World Applications of Cryo-Electron Microscopy
Drug Discovery and Design:
Cryo-electron microscopy has become an invaluable tool for drug discovery, especially in mapping drug targets such as membrane proteins and enzymes. Pharmaceutical companies rely on cryo-EM to visualize the structure of complex biomolecules at atomic resolutions, which is crucial for designing more effective drugs. Membrane proteins, which are key players in many diseases, were once difficult to image, but cryo-electron microscopy has changed that, leading to advances in the development of treatments for diseases like cancer and Alzheimer’s.
Virology:
Cryo-electron microscopy has proven essential in understanding viruses, including HIV, Zika, and SARS-CoV-2, the virus responsible for the COVID-19 pandemic. By using cryo-EM, scientists have been able to visualize viral structures and interactions with host cells in unprecedented detail. This has facilitated the development of vaccines and antiviral treatments. How does cryo-EM work in this context? It captures frozen samples of viruses, providing detailed snapshots of their molecular structure, which can then be used to design therapies that block viral replication.
Structural Biology:
In structural biology, cryo-electron microscopy has revolutionized the study of biomolecules, allowing researchers to capture previously impossible structures to image with traditional techniques like X-ray crystallography. This includes highly dynamic proteins and protein complexes, which play a crucial role in biological processes. The ability of cryo-EM to capture molecules in their native environment is a game-changer, revealing insights into how proteins function at a molecular level. So, how does cryo-EM work? It enables high-resolution 3D reconstructions of biological molecules in a frozen, hydrated state, providing a closer look at molecular mechanisms that drive life.
The Future of Cryo-EM: Innovations on the Horizon
Emerging Trends:
As technology advances, so do the capabilities of cryo-electron microscopy. Some of the latest trends include higher-resolution imaging, which allows for even more detailed views of molecular structures, and automated image processing. The integration of AI in image reconstruction is another frontier for cryo-EM, speeding up the process of analyzing complex biological structures. In the future, AI-driven systems may be able to handle large datasets more efficiently, enabling scientists to extract more meaningful insights from cryo-electron microscopy sample preparation and imaging.
Access and Cost:
As the technology matures, the cost of cryo-electron microscopy is gradually decreasing, making it more accessible to institutions and researchers worldwide. More facilities are investing in cryo-EM, which helps spread the availability of this powerful tool. However, cryo-electron microscopy sample preparation remains a costly and delicate process, requiring specialized equipment and trained personnel. Continued advancements in both equipment and techniques could help reduce costs and increase its usage, democratizing access to this transformative technology.
Future Potential:
The future of cryo-electron microscopy looks bright, with the potential for breakthroughs in medicine and biology. Researchers speculate that cryo-EM could be used to image single molecules, opening new avenues for personalized medicine and molecular diagnostics. Combining cryo-EM with machine learning may further accelerate drug discovery, enabling faster and more accurate identification of potential drug candidates. As cryo-electron microscopy sample preparation improves, it will enable the imaging of even more complex biological systems, pushing the boundaries of what we can see and understand.
Conclusion: The Lasting Impact of Cryo-Electron Microscopy
Cryo-electron microscopy has dramatically altered the landscape of molecular biology and medicine, enabling scientists to visualize previously inaccessible structures with incredible detail. From drug discovery to virology and structural biology, cryo-EM continues to revolutionize research fields that rely on high-resolution imaging of biomolecules. The innovation behind cryo-electron microscopy sample preparation has also been pivotal, ensuring that biological samples remain in their natural state during imaging. What is cryo-electron microscopy if not a game-changer? This technology has reshaped scientific research and is poised to continue doing so for years to come.
For those interested in the latest developments in cryo-electron microscopy, staying informed is essential. Whether you’re a scientist, student, or simply someone intrigued by cutting-edge technology, cryo-EM breakthroughs are worth following closely. Be sure to monitor how cryo-EM continues to impact biological and medical research, particularly in drug discovery and virology.
Check out the following interesting articles: