Introduction to Microfluidic Devices
Microfluidic devices are innovative systems that enable precise manipulation of fluids at the microscale, often handling volumes as small as nanoliters. These devices are pivotal in biomedical and nanotechnology applications due to their high efficiency, scalability, and cost-effectiveness. They operate through networks of microfluidic channels, where fluids are controlled using pressure gradients, pumps, or electric fields.
The history of microfluidics can be traced back to the 1980s, with its roots in microelectronics and inkjet printing. Today, Micro-scale fluidic devices play a critical role in diagnostics, drug delivery, and environmental monitoring, revolutionizing various scientific and industrial processes. For instance, the ability to achieve laminar flow (non-turbulent parallel fluid layers) enables precise chemical reactions, sample separations, and biological assays within these systems.
By addressing challenges such as reagent minimization and enhancing portability, these devices are transforming fields like clinical diagnostics and personalized medicine. This scalability and accuracy make microfluidics chips integral in developing tools like point-of-care diagnostic systems and lab-on-a-chip devices.
Microfluidics Chip: A Core Component
The microfluidics chip is the centerpiece of microfluidic systems, often crafted from materials like polydimethylsiloxane (PDMS), glass, or silicon. These materials are chosen for their biocompatibility, transparency, and ease of fabrication. Chips are created using advanced techniques such as photolithography, injection molding, or 3D printing.
Each chip contains a complex network of microfluidic channels designed to facilitate fluid mixing, separation, or reaction under precisely controlled conditions. For example, lab-on-a-chip devices integrate multiple laboratory functions—such as DNA amplification or cell sorting—into a single chip, significantly reducing analysis time and reagent consumption.
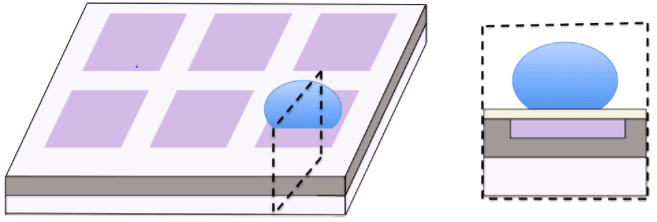
Applications of microfluidics chips include:
- Point-of-Care Diagnostics: Compact, portable chips enable rapid blood tests or infectious disease detection at the patient’s location. Examples include Abbott’s i-STAT system, which delivers real-time results in minutes.
- Droplet Microfluidics: These chips generate and manipulate microdroplets, essential for high-throughput drug discovery or single-cell analysis.
- Organs-on-Chips: Mimicking human organ functions, these chips provide a controlled environment for drug testing and disease modeling.
The versatility and precision of microfluidics chips underscore their importance in advancing biomedical research and nanotechnology
Microfluidic Channels: Engineering Marvels
Microfluidic channels are the foundational components of microfluidic devices, enabling precise fluid manipulation at the microscale. These channels are meticulously engineered to maintain laminar flow, ensuring non-turbulent and predictable fluid movement. This characteristic is critical for applications requiring high precision, such as chemical analysis, biological assays, and nanoparticle synthesis.
Fabrication Techniques
The design and fabrication of microfluidic channels rely on advanced techniques, such as:
- Photolithography: A process where UV light is used to etch channel patterns on a substrate.
- Soft Lithography: Often applied to PDMS, this method involves creating molds for flexible and biocompatible channels.
- 3D Printing: Provides rapid prototyping of intricate channel designs.
- Laser Ablation: Allows direct carving of channels on a variety of materials, such as glass or polymers.
The materials used, such as PDMS, silicon, or glass, are selected for their optical transparency, chemical resistance, and compatibility with biological samples. These properties enable precise monitoring of fluid behavior and ensure minimal contamination.
Key Applications
- Cell Sorting: Microfluidic channels are used to separate cells based on size, shape, or electrical properties. For example, fluorescence-activated cell sorting (FACS) integrated into microfluidics provides rapid and accurate cell separation for cancer research.
- Chemical Analysis: In analytical chemistry, these channels enable the separation of complex mixtures through techniques like micro-capillary electrophoresis.
- Nanoparticle Synthesis: Uniform nanoparticle production is achieved by controlling reaction parameters within channels, essential for drug delivery systems or imaging agents.
The microfluidic channels in these systems are indispensable for maintaining fluid precision, particularly in microfluidics chips for biomedical diagnostics. Their ability to handle minuscule fluid volumes while ensuring accuracy underscores their engineering brilliance.
Microfluidic Devices for Biomedical Applications
Microfluidic devices for biomedical applications are transforming the landscape of healthcare by enabling faster, more accurate diagnostics, personalized medicine, and innovative research platforms like organ-on-chip systems.
Diagnostic Applications
- Pathogen Detection: These devices facilitate the rapid identification of viruses, bacteria, or other pathogens using miniature diagnostic kits. For instance, COVID-19 detection kits based on microfluidic technology showcased high efficiency and portability.
- Biomarker Analysis: Devices equipped with microfluidic channels allow the analysis of biomarkers in bodily fluids like blood or saliva, aiding early disease detection.
Organ-on-Chip Platforms
Organ-on-chip systems, built using microfluidics chips, replicate the physiological environment of human organs. Examples include:
- Liver-on-Chip: Used to study drug metabolism and toxicity in a controlled environment.
- Lung-on-Chip: Simulates breathing motions and gas exchange, aiding respiratory disease research.
- Heart-on-Chip: Allows testing of cardiac drugs by mimicking heartbeat mechanics.
These platforms reduce reliance on animal testing and provide a closer approximation of human responses to treatments.
Broader Applications in Drug Testing
The precision of Micro-scale fluidic devices enables high-throughput screening of drug candidates. By integrating multiple processes into a single device, they reduce costs and increase the efficiency of pharmaceutical research.
“Microfluidic devices for biomedical applications have redefined healthcare by enabling efficient blood analysis, rapid pathogen detection, and advanced organ-on-chip studies. These tools bridge the gap between laboratory research and real-world medical applications, showcasing the versatility of microfluidic channels in transforming diagnostics and treatment development.”
Microfluidic Devices in Nanotechnology
The integration of Micro-scale fluidic devices in nanotechnology has revolutionized the precision and efficiency of nanoscale processes. These devices provide unparalleled control over fluid flow, enabling applications like nanoparticle synthesis and advanced biosensing technologies.
Integration with Nanotechnology
Microfluidic systems are particularly advantageous for nanotechnology due to their ability to manipulate tiny fluid volumes. This integration supports:
- Nanoparticle Fabrication: Precise control over reaction parameters such as temperature, flow rates, and mixing ensures uniform nanoparticle size and shape. For example, gold nanoparticles for medical imaging can be synthesized with high precision using Micro-scale fluidic devices.
- Biosensing Applications: Microfluidics enhances biosensors’ sensitivity and speed by enabling the capture and detection of biomolecules at the nanoscale. Devices equipped with microfluidics chips can detect DNA, proteins, or pathogens in real time.
- Nanoassembly: The controlled environment of microfluidic channels is ideal for self-assembling nanostructures like quantum dots or nanotubes, crucial in electronics and photonics.
Cutting-Edge Research
Recent advancements have highlighted the synergy between microfluidics and nanotechnology:
- Drug Delivery: Nano-carriers created using microfluidic methods ensure targeted and sustained drug release.
- Lab-on-a-Chip Devices: Combining nanomaterials with microfluidics chips has led to portable devices capable of diagnosing diseases at a molecular level.
“The use of microfluidic devices in nanotechnology has opened doors to precise nanostructure synthesis and advanced biosensing applications. By leveraging microfluidics chips, researchers can fabricate nanoparticles and develop innovative solutions for medical, environmental, and industrial challenges.”
Fundamental Concepts of Microfluidics
To understand what Micro-scale fluidic devices are, it’s essential to grasp the principles that govern fluid behavior at the microscale. Microfluidics operates on unique physical phenomena distinct from those observed at larger scales, making these devices indispensable in modern science and technology.
Core Principles
- Laminar Flow: Unlike turbulent flow at larger scales, microscale fluids exhibit smooth, predictable movement. This characteristic is vital for precision in mixing and reaction control within microfluidic channels.
- Capillary Forces: The dominance of surface tension over gravitational forces in microfluidics ensures efficient fluid transport and manipulation.
- Scaling Laws: At the microscale, physical forces such as viscosity and diffusion behave differently, influencing the design of microfluidics chips.
Active vs. Passive Microfluidic Systems
- Passive Systems: Relies on natural forces like capillarity or gravity. These are simple, cost-effective, and commonly used for capillary action in diagnostics.
- Active Systems: Utilize external forces like electric fields, pressure, or magnetic fields for precise control. Examples include electrophoresis or magnetophoresis within microfluidic devices.
“To fully appreciate what microfluidic devices are, one must delve into the principles of microscale physics. Concepts like laminar flow, capillary forces, and the behavior of fluids in microfluidic channels underpin their groundbreaking applications in science and medicine.”
Challenges and Future Directions
The development and application of microfluidic devices face several hurdles that must be addressed to unlock their full potential. At the same time, advancements in materials, integration technologies, and computational tools are paving the way for transformative innovations.
Challenges in Microfluidic Devices
- Scalability: While microfluidic systems excel in controlled laboratory settings, scaling up for industrial production poses challenges. Achieving consistent results across high-volume manufacturing requires novel fabrication methods.
- Material Limitations: Common materials like PDMS and glass offer advantages such as transparency and biocompatibility. However, they may lack durability or introduce unwanted interactions with biological samples. Alternatives like thermoplastics or hybrid materials are under exploration.
- Complexity in Fabrication: Creating intricate microfluidic channels and incorporating multiple functionalities in a single chip remains technically demanding.
- Integration with Existing Technologies: Merging microfluidic systems with other technologies, such as biosensors or nanotechnology platforms, requires seamless engineering and compatibility testing.
Future Opportunities
- AI and Machine Learning Integration: By incorporating AI algorithms, microfluidic devices can process and analyze data in real time, enabling personalized medicine and precision diagnostics.
- Advances in Personalized Medicine: Microfluidics is set to play a key role in tailoring treatments based on an individual’s unique genetic and biochemical profile.
- Nanotechnology Synergies: The intersection of microfluidic devices in nanotechnology offers opportunities for designing highly sensitive diagnostic tools and efficient drug delivery systems.
- Sustainability: Emerging efforts aim to use eco-friendly materials and energy-efficient manufacturing processes, reducing the environmental impact of these devices.
The advancement of Micro-scale fluidic devices hinges on addressing material limitations and scalability issues. With advancements in AI and microfluidic devices for biomedical applications, these systems are poised to revolutionize diagnostics and therapeutic solutions.”
Conclusion
Micro-scale fluidic devices have emerged as a cornerstone in the fields of biomedicine and nanotechnology. From the intricacies of microfluidic channels to the cutting-edge applications in diagnostics and nanoparticle synthesis, their impact is profound.
As the technology evolves, addressing challenges like scalability and material limitations will be critical. The integration of AI, personalized medicine, and sustainable practices signifies a promising future.
Call to Action
“To fully harness the potential of microfluidic devices, ongoing research and innovation are essential. Whether you’re a scientist, an engineer, or a healthcare professional, exploring the applications and future opportunities of this transformative technology could unlock new possibilities.”
Additional Resources and Next Steps
Microfluidic devices have reshaped the landscape of biomedical applications and nanotechnology, providing scalable and efficient solutions for diagnostics, drug delivery, and more. To explore related topics and deepen your understanding, check out the following resources:
Explore how lab-on-a-chip innovations streamline diagnostics and enable precise, rapid results for healthcare and research.
Learn how assistive technologies have advanced to support individuals with disabilities, integrating innovations like microfluidic solutions.
Discover how cutting-edge imaging techniques complement microfluidic research in understanding complex brain functions.
Trace the history of medical technologies, from early tools to modern breakthroughs like microfluidic devices.