🧬 Introduction: What Are MXenes and Why Are They Important?
In the ever-evolving world of nanotechnology, MXenes have emerged as a groundbreaking class of 2D materials with immense potential across energy, electronics, and environmental applications. First discovered in 2011 by researchers at Drexel University, MXenes are derived from a larger group of layered ceramics known as MAX phases—named for their composition of transition metals (M), aluminum or other group A elements (A), and carbon/nitrogen (X).
Structurally, MXenes materials are composed of 2–3 atom-thick layers of transition metal carbides, nitrides, or carbonitrides. They are created by selectively etching the A-element from their MAX-phase precursor, leaving behind sheets of conductive ceramics with unique surface terminations like –O, –OH, or –F. These terminations allow these 2D materials to exhibit excellent hydrophilicity, high capacitance, and remarkable electrical conductivity—making them highly attractive for real-world technological advancements.

⚖️ MXenes vs Graphene: A Crucial Comparison
While graphene continues to dominate headlines as the most famous 2D material, MXenes materials offer distinct advantages:
- Hydrophilicity: MXenes disperse easily in water due to their surface groups, unlike the hydrophobic nature of pristine graphene.
- Surface Modifiability: MXenes can be chemically tuned for specific properties, enhancing their role in electrochemical and catalytic applications.
- Conductivity: Although graphene boasts extremely high conductivity, MXenes exhibit comparable electrical conductivity while also providing tunable surface chemistry.
- Scalability: MXenes can be synthesized from relatively abundant MAX phases, potentially making them more accessible for industrial production.
This combination of metallic conductivity, flexibility, and functional surface chemistry positions titanium-carbide-based material as a superior alternative to many traditional and modern 2D materials, especially in fields like energy storage, EMI shielding, and wearable electronics.
As the global push for high-performance, low-footprint materials accelerates, the versatility and tunability of MXenes materials may shape the next frontier of nanotech and functional ceramics.
🧱 Understanding the MAX Phase: MXenes’ Parent Compound
To fully grasp the significance of MXenes, it’s essential to delve into their origins—the MAX phases. These 2D layered compounds serve as the foundational precursor materials from which MXenes are synthesized. Understanding the structure, properties, and synthesis of MAX phases illuminates the transformative journey to MXenes.
🔬 Structure and Composition of MAX Phases
MAX phases are a unique class of ternary carbides and nitrides with the general formula Mₙ₊₁AXₙ, where:
- M represents an early transition metal (e.g., Titanium (Ti), Vanadium (V), Niobium (Nb)).
- A denotes a group 13 or 14 element (commonly Aluminum (Al), Silicon (Si)).
- X stands for Carbon (C) and/or Nitrogen (N).
- n is an integer (1, 2, or 3) indicating the number of M-X layers.
These compounds crystallize in a hexagonal structure characterized by alternating layers of:
- M-X blocks: n+1 layers of transition metal (M) interleaved with n layers of carbon or nitrogen (X).
- A layers: Single atomic layers of the A element separating the M-X blocks.
This layered architecture imparts MAX phases with a distinctive combination of metallic and ceramic properties, including:
- High electrical and thermal conductivity (metallic trait).
- Mechanical robustness and damage tolerance (ceramic trait).
- Excellent machinability.
- Resistance to oxidation and thermal shock.
These attributes make MAX phases versatile in applications such as high-temperature coatings, electrical contacts, and structural components.
🔄 Transition from MAX Phase to MXene: The Role of Selective Etching
The transformation of MAX phases into titanium-carbide-based material involves a selective etching process, wherein the A element is removed, typically using chemical agents. This process unfolds as follows:
- Selective Etching: The MAX phase powder is immersed in an etching solution (commonly hydrofluoric acid (HF) or a mixture like LiF-HCl), which selectively dissolves the A layers, breaking the M-A bonds and liberating the M-X layers.
- Formation of MXene Sheets: The removal of A layers results in the formation of 2D layers of transition metal carbides or nitrides, now termed MXenes. These layers inherit the structural integrity of the original M-X blocks but exhibit new surface chemistries due to the termination groups introduced during etching (e.g., –OH, –O, –F).
- Delamination: To achieve single or few-layer MXene sheets, the etched material can undergo further delamination, often assisted by sonication or intercalation with organic molecules, enhancing their dispersion in solvents and usability in various applications.
🧪 Advancements in Etching Techniques
While HF etching is prevalent, concerns about safety and environmental impact have spurred the development of alternative methods:
- In Situ HF Generation: Utilizing safer fluoride-containing salts (e.g., ammonium bifluoride) to generate HF in situ, reducing handling risks.
- Molten Salt Etching: Employing molten salts like potassium fluoride (KF) for etching, which can offer more controlled reactions and potentially yield MXenes with different surface terminations.
- Alkali Etching: Recent studies have explored hydrothermal etching using alkali solutions (e.g., potassium hydroxide), providing a fluoride-free route to MXene synthesis.
These advancements aim to enhance the safety, efficiency, and environmental friendliness of MXene production.
🧩 Visualizing the Transformation: From MAX to MXene
To aid in understanding, consider an infographic illustrating:
- Atomic Structure of MAX Phase: Depicting the alternating M-X and A layers.
- Etching Process: Showing the selective removal of A layers.
- Resulting MXene Structure: Highlighting the separated M-X layers with surface terminations.
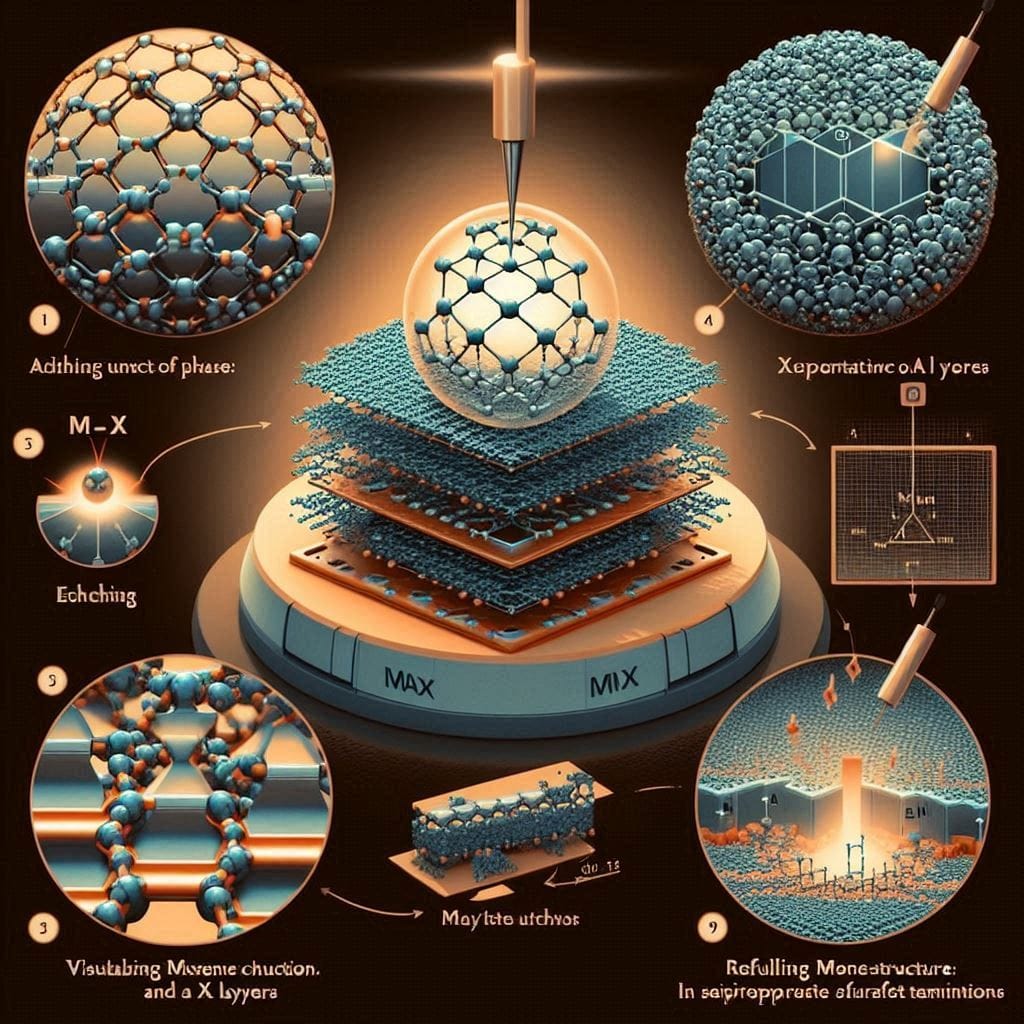
Such visual aids can effectively convey the structural evolution during the MAX to MXene transformation.
⚗️Synthesis of MXenes: Methods and Challenges
The synthesis of MXenes primarily involves the selective etching of A-group elements from their parent MAX phases, resulting in the formation of two-dimensional transition metal carbides or nitrides. This section delves into the various synthesis techniques, their efficiencies, safety considerations, and associated costs.
🧪 Selective Etching Techniques
Hydrofluoric Acid (HF) Etching:
- Process: Direct immersion of MAX phase powders in HF leads to the removal of A layers, yielding multilayered 2D materials.
- Advantages: Effective and straightforward.
- Challenges: HF is highly toxic and poses significant handling risks.(future prospect of Mxenes)
Safer Alternatives (e.g., LiF + HCl):
- Process: A mixture of lithium fluoride (LiF) and hydrochloric acid (HCl) generates in situ HF, facilitating etching.
- Advantages: Offers a balance between safety and efficiency; reduces direct handling of HF.
- Challenges: Requires precise control over reaction conditions.(ScienceDirect)
Molten Salt Etching:
- Process: Utilizes molten salts to etch MAX phases at elevated temperatures.
- Advantages: Produces it with minimal surface terminations, potentially enhancing electrical conductivity.(RSC Publishing)
- Challenges: High-temperature requirements and potential material degradation.
🌀 Delamination Techniques
Post-etching, obtaining single or few-layer MXene sheets involves delamination:
- Intercalation with Organic Molecules: Compounds like dimethyl sulfoxide (DMSO) are intercalated between MXene layers, followed by sonication to achieve separation.
- Mechanical Agitation: Direct sonication without intercalants can also delaminate these 2D materials but may yield lower concentrations of single-layer sheets.
♻️ Green Synthesis Approaches
Efforts toward environmentally friendly MXene synthesis focus on:MDPI
- Fluoride-Free Etching: Exploring etchants like sodium hydroxide (NaOH) to eliminate the need for hazardous fluorine-containing chemicals.
- Electrochemical Etching: Utilizing electrochemical methods to selectively remove A layers, reducing chemical waste.
- Gas-Phase Etching: Employing gaseous etchants to minimize liquid waste and simplify purification processes.
MPDI-Sustainable Synthesis of MXenes
📊 Comparison of Synthesis Techniques
| Method | Efficiency | Safety | Cost |
|---|---|---|---|
| HF Etching | High | Low (toxic and hazardous) | Moderate |
| LiF + HCl Etching | Moderate | Higher (safer handling) | Moderate |
| Molten Salt Etching | Moderate | Moderate (high-temp) | High (energy-intensive) |
| Green Methods | Varies | High (eco-friendly) | Variable |
🏗️ MXenes Structure: Composition, Morphology & Terminations
Understanding the structure of MXenes is crucial, as their composition, morphology, and surface terminations significantly influence their properties and applications.
🧬 Composition and Morphology
These 2D materials are characterized by their two-dimensional layered structure, consisting of:
- Transition Metal Layers (M): Early transition metals like titanium (Ti), molybdenum (Mo), or vanadium (V).
- Carbon and/or Nitrogen Layers (X): Carbides, nitrides, or carbonitrides forming strong M-X bonds.
The layers are held together by van der Waals forces, allowing for intercalation and delamination.
🎯 Surface Terminations (Tₓ Groups)
During synthesis, MXenes acquire various surface terminations, commonly denoted as Tₓ, including:
- –OH (Hydroxyl) Groups
- –O (Oxygen) Groups
- –F (Fluorine) Groups
These terminations arise from the etching process and significantly affect MXenes’ properties.
🌊 Role of Terminations in Hydrophilicity and Conductivity
- Hydrophilicity: The presence of –OH and –O groups enhances MXenes’ affinity for water, making them highly dispersible in aqueous solutions. This property is advantageous for processing and integration into composites.
- Electrical Conductivity: While these 2D materials are inherently conductive, surface terminations can influence their conductivity. For instance, –O terminations have been associated with higher conductivity compared to –F terminations, which may introduce scattering centers that reduce electron mobility.
🚀 MXenes Applications: Current and Emerging Uses
These layered nanomaterials, a class of two-dimensional (2D) transition metal carbides and nitrides, have garnered significant attention due to their exceptional properties, including high electrical conductivity, hydrophilicity, and mechanical strength. These attributes make them highly versatile for various applications across multiple domains.
Energy Storage
MXenes have emerged as promising materials in the field of energy storage, particularly in batteries and supercapacitors, due to their excellent electrical conductivity and large surface area.
Batteries (Li-ion, Na-ion, Zn-ion):
- Lithium-Ion Batteries (LIBs): MXenes serve as efficient anode materials in LIBs, offering high capacity and rapid charge-discharge capabilities. Their layered structure facilitates lithium-ion intercalation, enhancing battery performance.
- Sodium-Ion Batteries (SIBs): Given the abundance of sodium, SIBs are gaining interest. Nanolaminates contribute to SIB development by providing stable and high-capacity anode materials.
- Zinc-Ion Batteries (ZIBs): MXenes are explored as cathode materials in ZIBs, demonstrating high capacity and stability, which are crucial for the advancement of aqueous rechargeable batteries.
Supercapacitors:
Nanolaminates are extensively studied for supercapacitor applications due to their high conductivity and surface area. They enable devices with rapid charge-discharge cycles and long-term stability, making them suitable for applications requiring quick energy bursts.
🌍 Environmental Applications
The unique surface chemistry and high adsorption capacity of MXenes make them effective in environmental remediation efforts. (ScienceDirect- Application of Mxenes in Environmental remediation)
Water Purification:
MXenes exhibit excellent potential in water treatment processes. Their hydrophilic nature and large surface area allow for effective removal of contaminants, including heavy metals and organic pollutants, from water sources.
Heavy Metal Adsorption:
MXenes can adsorb heavy metal ions such as lead (Pb²⁺), mercury (Hg²⁺), and chromium (Cr³⁺) from aqueous solutions. This capability is attributed to their negatively charged surfaces and functional groups, which facilitate the binding of positively charged metal ions.
📡 Sensors & Electronics
The exceptional electrical properties and mechanical flexibility of MXenes position them as ideal candidates for advanced sensor and electronic applications.
Flexible and Wearable Electronics:
MXenes are utilized in the development of flexible and wearable electronic devices. Their mechanical robustness and conductivity enable the creation of sensors that conform to various surfaces, suitable for health monitoring and human-machine interfaces.
Gas Sensors:
MXene-based gas sensors demonstrate high sensitivity and selectivity towards various gases, including ammonia (NH₃), acetone, and nitrogen dioxide (NO₂). Their large surface area and tunable surface chemistry enhance gas adsorption and detection capabilities.
🛡️ EMI Shielding & Conductive Coatings
MXenes are explored for electromagnetic interference (EMI) shielding and conductive coating applications, particularly in aerospace and defense sectors.
Lightweight Materials for Aerospace and Defense:
The high conductivity and lightweight nature of MXenes make them suitable for EMI shielding in aerospace and defense applications. They offer effective protection against electromagnetic pollution, ensuring the proper functioning of electronic equipment.
Performance Metrics Comparison:
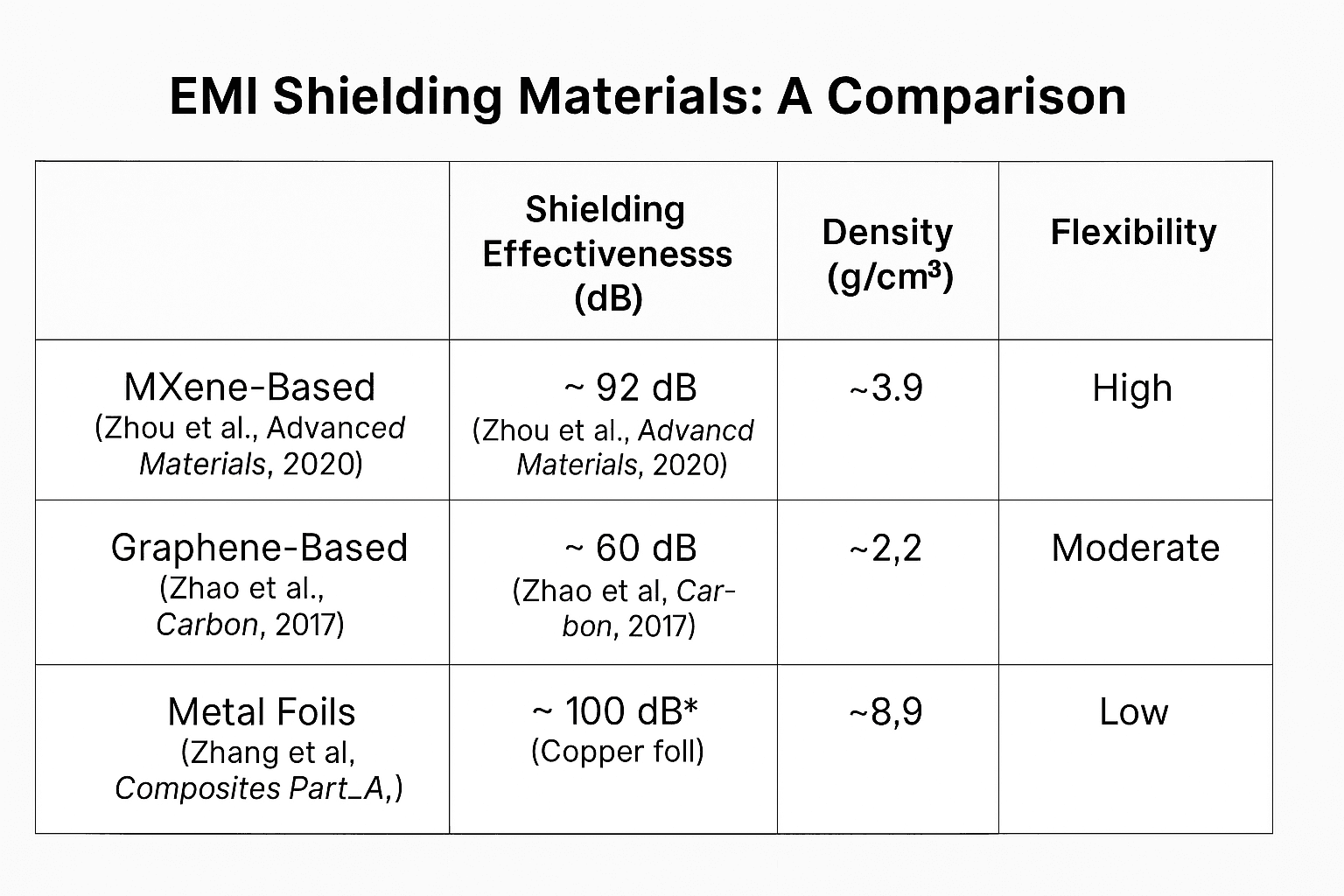
-
Shielding Effectiveness is measured typically in the 8–12 GHz range.
-
MXenes like Ti₃C₂Tₓ have shown very high EMI shielding due to conductivity and layered structure.
-
Graphene composites provide moderate shielding with better weight and flexibility than metals.
-
Metal foils like copper are highly effective but heavy and less flexible.
This table illustrates that MXene-based materials offer a balanced combination of high shielding effectiveness, low density, and flexibility, making them advantageous over traditional materials in specific applications.
⚖️ Advantages and Limitations of MXenes
MXenes, a family of two-dimensional (2D) transition metal carbides and nitrides, have garnered significant attention due to their unique properties. However, like all materials, they possess both advantages and limitations that influence their applicability across various domains.
✅ Advantages of MXenes
High Electrical Conductivity:
- It exhibit exceptional electrical conductivity, with values reaching up to 20,000–24,000 S/cm for pure films. This property makes them highly suitable for applications in energy storage devices and electronic components.
Tunable Surface Chemistry:
- The surface of these 2D materials can be functionalized with various groups (e.g., –OH, –O, –F), allowing for the customization of their chemical properties to suit specific applications. This tunability enhances their versatility in fields such as catalysis and sensing.
Mechanical Strength and Flexibility:
- MXenes possess robust mechanical properties, combining strength with flexibility. This combination is advantageous for developing flexible electronics and wearable devices.
❌ Limitations of MXenes
Oxidation Stability:
- MXenes are prone to oxidation when exposed to environmental conditions, leading to degradation of their properties over time. Enhancing their environmental stability remains a critical challenge for their practical application. SpringerLink
Complexity of Synthesis:
- Traditional synthesis methods of MXenes often involve hazardous chemicals like hydrofluoric acid, posing safety and environmental concerns. Developing safer and more efficient synthesis techniques is essential for their large-scale production.
🗣️ Expert Insights
Dr. Yury Gogotsi, a leading researcher in the field of 2D materials, emphasizes the importance of addressing these challenges:
“While MXenes offer a plethora of advantageous properties, overcoming their susceptibility to oxidation and refining synthesis methods are pivotal for their transition from laboratory research to industrial applications.”
🔮 Future Outlook: What’s Next for MXenes?
The future of MXenes is poised at the intersection of advanced computational techniques and scalable manufacturing processes, promising to unlock new potentials in material science.
🤖 AI-Assisted Material Discovery
Artificial Intelligence (AI) is revolutionizing the way new materials are discovered and optimized:
Predictive Modeling:
- Machine learning algorithms can predict the properties of MXenes based on their composition and structure, accelerating the design of materials with tailored functionalities.
Optimization of Synthesis Parameters:
- AI facilitates the optimization of synthesis conditions, enhancing yield and quality while minimizing the need for extensive trial-and-error experiments.
🏭 Industrial-Scale Synthesis Techniques
Transitioning these 2D materials from the laboratory to commercial applications necessitates the development of scalable and environmentally friendly synthesis methods:
Fluorine-Free Etching Processes:
- Researchers are exploring alternative etching methods that eliminate the use of hazardous fluorine-containing chemicals, improving safety and sustainability.
Mechanochemical Approaches:
- Utilizing mechanical forces to drive chemical reactions offers a solvent-free route to synthesize MXenes, aligning with green chemistry principles.
🚀 Innovators Driving Progress
Several startups and research institutions are at the forefront of MXene innovation:
Drexel University:
- As the birthplace of MXenes, Drexel continues to lead in research and development, focusing on expanding the MXene family and exploring new applications.
Nanomaterials Institute Spin-offs:
- Companies emerging from academic research are working on translating MXene technology into commercial products, particularly in energy storage and electronic applications.
🧪 Conclusion: The Future of MXenes in Science and Technology
MXenes represent one of the most promising frontiers in nanomaterials and 2D material science. With their exceptional electrical conductivity, mechanical flexibility, hydrophilicity, and tunable surface chemistry, they are uniquely suited for a broad spectrum of applications—from energy storage and electromagnetic shielding to biomedical devices and sensors.
However, real-world adoption still faces challenges. Oxidation stability, scalable synthesis methods, and the environmental impact of production are key research areas that require further exploration. Encouragingly, global interest is accelerating, with research institutions, startups, and industrial partners investing heavily in MXene-related innovations.
As synthesis techniques become more refined and application-specific MXenes are developed, we are likely to witness their integration into commercial technologies, driving breakthroughs in flexible electronics, sustainable energy, and next-gen materials.
More Articles You Might Like:
-
🔬 What is Graphene? A One-Atom-Thick Marvel – Dive into the revolutionary material that sparked the 2D materials revolution.
-
🧪 Graphene Biosensors Explained – Discover how graphene is transforming the world of medical diagnostics and biosensing.
-
🔄 Liquid Crystal Elastomers – Learn about these shape-shifting materials powering the next-gen soft robotics.
-
🧬 Unlocking Self-Assembling Materials – Explore materials that build themselves—opening doors to futuristic applications.