Introduction: The Next Frontier in Cancer Treatment
What if cancer could be prevented—or even treated—with a simple shot?
It’s a question that once belonged only in the realm of science fiction. But today, thanks to the remarkable progress in cancer vaccine development, this bold vision is inching closer to reality.
At the forefront of this innovation is the mRNA cancer vaccine—a revolutionary approach that uses personalized genetic instructions to teach the body’s immune system how to recognize and destroy cancer cells. Unlike traditional therapies that aim to kill tumors directly, this technique trains your immune system to do the job, offering a precision-driven, long-lasting solution.
In early human trials, mRNA-based vaccines have shown promising results against some of the most aggressive cancers, including lung, breast, and pancreatic cancers. These aren’t just generic cancer vaccines; they’re personalized immunotherapies—tailored to the genetic makeup of a patient’s individual tumor.
This blog explores how mRNA cancer vaccine technology works, why it could transform the future of immunotherapy, and what makes it such a significant leap in the fight against cancer.
🧬 Key Highlights We’ll Cover:
- How mRNA vaccines are designed to target tumor-specific mutations
- The clinical trial results giving researchers hope
- Benefits of personalized cancer immunotherapy
- Challenges ahead in regulatory approval, scalability, and public access
🧬 How mRNA Cancer Vaccines Work
Understanding how mRNA cancer vaccines work doesn’t require a degree in molecular biology—it just requires the right metaphor: Imagine giving your immune system a “wanted poster” of cancer cells so it can find and destroy them.
That’s exactly what these vaccines do.
🧬 What Is an mRNA Cancer Vaccine?
At its core, an mRNA cancer vaccine is a personalized cancer immunotherapy. Instead of using a live virus or protein, it uses messenger RNA (mRNA)—genetic instructions—to tell your body how to produce a protein that resembles a specific feature of a cancer cell (known as a tumor antigen).
Once the protein is made by your own cells, your immune system:
- Recognizes it as foreign or dangerous
- Builds a targeted response (usually by activating T-cells)
- Retains this immune memory to fight future recurrences
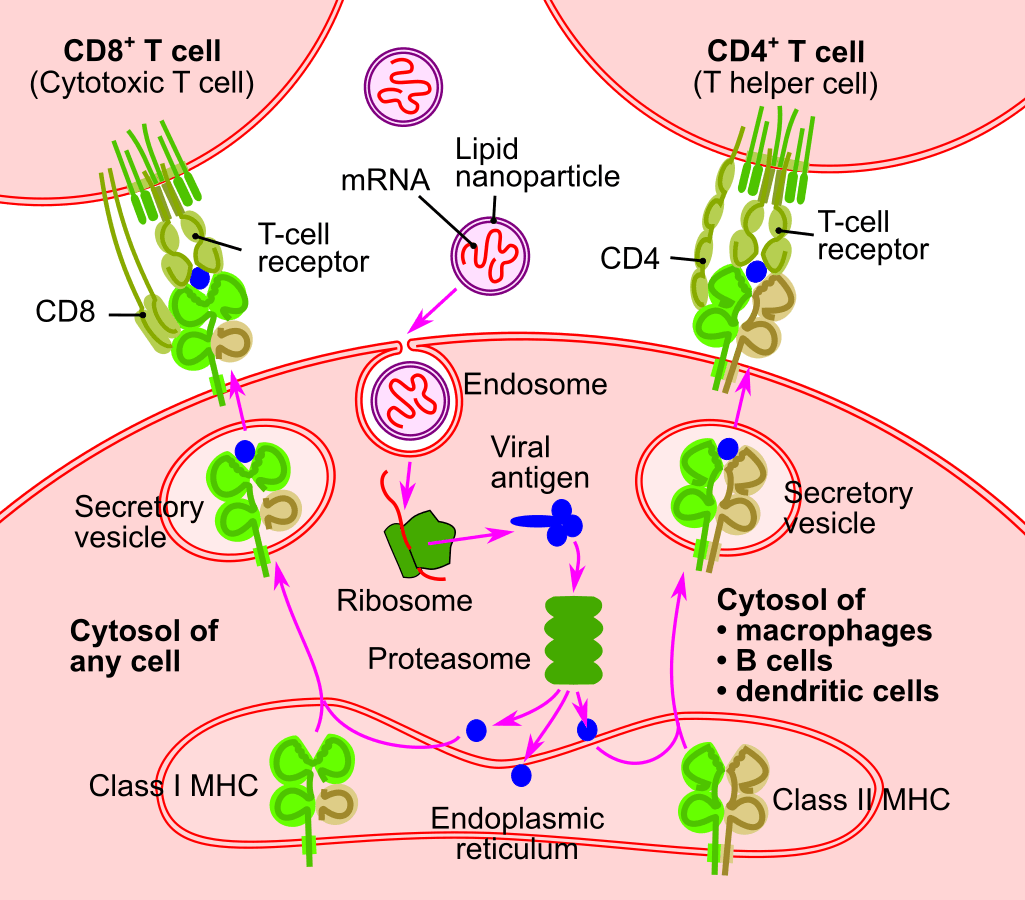
⚙️ Step-by-Step: How mRNA Vaccines Work
- Tumor analysis: Scientists first sequence the DNA of a patient’s tumor to identify specific mutations.
- mRNA design: They then create an mRNA strand that codes for proteins only found on the patient’s cancer cells.
- Injection: The mRNA is delivered into the patient’s body via a lipid nanoparticle (like in COVID vaccines).
- Cellular production: Body cells read the mRNA and produce the custom-designed protein.
- Immune activation: The immune system recognizes the protein and launches a targeted attack on cancer cells expressing it.
💡 In short: mRNA delivers the blueprint. Your cells make the decoy. Your immune system brings the firepower.
🧬 Comparison: mRNA Vaccines for COVID vs Cancer
| Feature | COVID-19 mRNA Vaccine | mRNA Cancer Vaccine |
|---|---|---|
| Target | Viral spike protein (SARS-CoV-2) | Tumor-specific proteins (neoantigens) |
| Design | Standardized for population | Personalized for each patient |
| Goal | Prevent infection | Treat or prevent cancer recurrence |
| Timeline | Mass-produced, pre-formulated | Tailor-made after tumor biopsy |
Both use the same delivery technology, but the mRNA cancer vaccine is highly individualized, making it one of the most precise tools in personalized medicine today.
🔬 Why This Approach Matters
Traditional cancer treatments like chemotherapy or radiation often damage healthy cells. In contrast, personalized cancer immunotherapy via mRNA ensures that only cancer cells are targeted, reducing side effects and potentially offering long-term immunity against tumor relapse.
This method could redefine how we treat aggressive cancers like lung, breast, and pancreatic—where early detection is rare and treatment options are limited.
🧪 The Human Trials: Hope for Lung, Breast & Pancreatic Cancer
The early results of mRNA clinical trials for cancer are delivering what many thought impossible: personalized cancer vaccines that show measurable success—even against some of the most difficult cancers like lung, breast, and pancreatic.
Leading biotech firms BioNTech (known for the Pfizer COVID vaccine) and Moderna are now advancing their mRNA platforms beyond infectious diseases into oncology, with some truly promising outcomes.
📊 Real Trial Results: Personalized mRNA Cancer Vaccines in Action
✅ BioNTech & Genentech: Phase I Pancreatic Cancer Trial
Study published: Nature, April 2023
Vaccine candidate: BNT122 (autogene cevumeran)
Study size: 16 patients with surgically removed pancreatic cancer
Outcome:
- 8 out of 16 patients (50%) showed strong T-cell responses to tumor-specific mutations.
- Those with immune responses remained cancer-free after 18 months, while others experienced recurrence.
Side effects: Mild to moderate flu-like symptoms; overall well-tolerated.
✅ Moderna & Merck: mRNA Vaccine for Melanoma (with future expansion to lung cancer)
Presented: AACR 2023 (American Association for Cancer Research)
Trial name: KEYNOTE-942
Patient count: 157
Result:
- Adding personalized mRNA vaccine to immunotherapy (Keytruda) reduced cancer recurrence by 44% compared to immunotherapy alone.
- Expansion plans underway for lung and breast cancer trials using similar technology.
💬 “These results open the door for personalized mRNA vaccines to transform cancer care,” said Uğur Şahin, CEO of BioNTech.
💉 Where It’s Showing the Most Promise
So far, mRNA vaccine trials in cancer are seeing the strongest early results in:
- Pancreatic cancer (BioNTech): Improved recurrence-free survival
- Melanoma (Moderna): Reduced relapse when combined with checkpoint inhibitors
- Lung cancer (early-stage trials starting in 2024): High potential due to well-known tumor antigens
- Breast cancer (preclinical, with patient-specific mutation targeting in development)
👩⚕️ Real Patient Highlight: A Glimpse of Success
One participant in BioNTech’s pancreatic cancer trial—diagnosed with one of the most lethal cancer types—remained cancer-free 18 months post-surgery, after mounting a robust immune response from the vaccine.
Although the data set is small, this result is statistically and clinically meaningful in a cancer type where survival rates are historically very low.
📣 Early results show that mRNA isn’t just a vaccine platform—it’s a personalized immune weapon against cancer.
🌟 Why This Breakthrough Matters
The arrival of personalized mRNA cancer treatment marks a pivotal shift in oncology—offering a new standard that could eventually redefine how we treat, manage, and even prevent cancer.
Unlike traditional cancer therapies that often take a one-size-fits-all approach, mRNA cancer vaccines offer precision, speed, and adaptability—qualities that make this technology not just innovative, but potentially transformative.
⚖️ mRNA vs Traditional Treatments: A Clear Advantage
💉 Compared to Chemotherapy
- Traditional chemotherapy indiscriminately attacks rapidly dividing cells—both cancerous and healthy—often resulting in harsh side effects like hair loss, fatigue, and immune suppression.
- In contrast, mRNA cancer vaccines are targeted: they train the immune system to attack only cells that display tumor-specific markers, minimizing damage to healthy tissues.
- The result? Lower toxicity, better patient tolerance, and improved quality of life during treatment.
🧬 Compared to CAR-T and Checkpoint Inhibitors
While CAR-T therapy and immune checkpoint inhibitors have revolutionized cancer immunotherapy, they come with limitations:
- Complex manufacturing processes
- Longer lead times
- High costs—often exceeding $300,000 per patient
mRNA cancer vaccines, on the other hand, can be produced more rapidly, customized to individual patients in weeks, and are potentially far more scalable for widespread clinical use.
🚀 Why It’s a True Cancer Immunotherapy Breakthrough
The strength of this breakthrough lies in three core advantages:
🛠️ Scalability
- Once the delivery platform (e.g., lipid nanoparticles) is established, mRNA sequences can be swapped in and out, allowing for rapid adaptation to different tumors or new mutations.
🩹 Low Toxicity
- By targeting only the tumor’s genetic fingerprints, mRNA cancer vaccines dramatically reduce the collateral damage seen in traditional therapies.
⏱️ Speed of Customization
- From biopsy to vaccine, the timeline could shrink to just a few weeks, enabling fast, adaptive responses—especially important for aggressive cancers.
🌍 Toward a Future Where Cancer Is Manageable—or Even Preventable
If early trial results continue to hold, mRNA-based personalized cancer vaccines could shift cancer care from reactive to proactive. Imagine:
- A future where a pancreatic tumor is removed and a custom vaccine prevents recurrence
- Or where genetic risk identifies someone as a candidate for a preventive mRNA shot before cancer ever develops
📣 Could this be the beginning of the end for cancer as we know it?
This is the promise of cancer immunotherapy breakthroughs driven by mRNA—not just treatment, but transformation.
🔭 What’s Next? Expanding Trials and Future Cancers
The early success of mRNA for cancer is just the beginning. Now that personalized cancer vaccines have proven safe and potentially effective in small-scale studies, the field is gearing up for larger trials, broader cancer targets, and accelerated integration into modern oncology.
The cancer vaccine pipeline is expanding quickly—and it’s attracting the attention of some of the world’s most advanced biotech firms.
🧪 The Road Ahead: From Proof to Practice
📈 Phase 2/3 Clinical Trials
The most immediate next step in the evolution of mRNA cancer vaccines is the initiation of Phase 2 and 3 trials. These trials will:
- Enroll hundreds to thousands of patients
- Compare mRNA vaccines against current standards of care
- Measure endpoints like recurrence-free survival, tumor regression, and overall survival
Moderna and Merck’s KEYNOTE-942 trial is already progressing toward Phase 3 for melanoma, with similar pathways likely for lung and breast cancer in 2024–2025.
🧬 Expansion to More Cancer Types
Beyond melanoma, pancreatic, lung, and breast cancers, researchers are developing mRNA-based immunotherapies for:
- Colorectal cancer (Moderna, Gritstone Bio)
- Ovarian cancer (CureVac)
- Glioblastoma (personalized neoantigen approaches under study)
- Bladder and kidney cancers (in combination trials)
The future of mRNA vaccines lies in this adaptability—the ability to personalize vaccines to any tumor with a unique mutational profile.
🤝 The Role of Combination Therapies
One promising direction is combining mRNA for cancer with existing immunotherapies like:
- Checkpoint inhibitors (e.g., Keytruda, Opdivo)
- T-cell therapies or immune modulators
The synergy could result in more durable, complete tumor responses, even in cancers that were previously considered treatment-resistant.
For example, the Moderna/Keytruda combo reduced recurrence by 44% in melanoma patients—a major leap over monotherapies.
🧪 Leading Players in the Race
Several companies are advancing their cancer vaccine pipelines:
| Company | Vaccine Focus | Notable Projects |
|---|---|---|
| BioNTech | Pancreatic, colorectal, melanoma | BNT122 (w/ Genentech) |
| Moderna | Melanoma, lung, breast | mRNA-4157 (KEYNOTE-942 trial) |
| CureVac | Ovarian, head & neck | Preclinical pipeline |
| Gritstone Bio | Colorectal, lung | Personalized neoantigen vaccine (SLATE) |
These firms are pushing the science forward with rapid design cycles, clinical scale-up, and global trial sites.
🧠 Bonus Insight: How AI Is Speeding It Up
One major innovation behind the scenes? Artificial intelligence.
AI models are now being used to:
- Map tumor DNA to identify the most immunogenic mutations
- Prioritize neoantigens that will provoke the strongest immune response
- Generate mRNA sequences in days, not months
This is shaving critical time off the vaccine development cycle, making it feasible to deliver custom cancer vaccines in just weeks.
💬 With AI and mRNA converging, the future of cancer care may soon be rapid, precise, and deeply personalized.
⚠️ Challenges and Limitations
While the excitement around mRNA cancer vaccines is justified, it’s essential to recognize the current limitations and ongoing challenges facing this transformative technology. As we move from successful early trials to widespread clinical use, researchers and policymakers alike must confront several scientific, logistical, and ethical hurdles.
💰 High Cost of Personalization
One of the major mRNA cancer vaccine limitations is cost. Unlike traditional vaccines, these are not mass-produced, one-size-fits-all solutions. Each dose is:
- Customized to a patient’s tumor mutations
- Requires DNA sequencing, computational design, and rapid mRNA synthesis
- Often paired with complex immunotherapy regimens
This process currently costs tens of thousands of dollars per patient, making scalability and affordability a major concern.
🧪 Still Early in the Development Pipeline
Despite promising trial results, most mRNA cancer vaccines are:
- In Phase 1 or 2 trials
- Not yet FDA-approved for standard clinical use
- Lacking long-term safety and efficacy data
While accelerated pathways may speed up progress, full regulatory approval is likely 2–5 years away, depending on trial outcomes.
🧬 Tumor Immune Evasion
Cancer is notoriously adept at hiding from the immune system. Some tumors:
- Mutate rapidly, making fixed vaccine targets obsolete
- Create immune-suppressive environments (e.g., T-cell exhaustion)
- Downregulate the expression of neoantigens the vaccine is meant to target
These biological escape mechanisms present a key cancer vaccine challenge, especially in advanced or resistant cancers.
🔬 Addressing the Roadblocks
Researchers are actively working to overcome these limitations through:
- Automation of mRNA design using AI and robotics
- Machine learning models that quickly identify viable neoantigen targets
Combination therapies, pairing mRNA vaccines with:
- Checkpoint inhibitors
- CAR-T cells
- Small molecule immune boosters
This multimodal approach could improve response rates and overcome immune suppression in stubborn cancers.
⚖️ Ethical and Access Concerns
As with all personalized cancer treatments, access and equity are growing concerns. Without deliberate planning, there’s a risk that:
- Only patients in wealthy countries or private systems may benefit
- Personalized vaccines become a luxury care option, not a standard of care
- Data and AI models used in design remain proprietary, slowing global adoption
🌍 Ensuring ethical access to mRNA cancer vaccines will be as critical as the science itself.
🎯 Conclusion: A Shot at a Cancer-Free Future?
The journey of cancer vaccine development has taken a revolutionary turn—with mRNA technology leading the way. What was once seen as a distant dream is now emerging as a real, science-backed possibility: a personalized shot that trains your immune system to identify and eliminate cancer cells, just as it would a virus.
The science is solid—decades of molecular biology and immunology have laid the foundation.
The human trial results are encouraging, showing real clinical benefits in tough-to-treat cancers like pancreatic, melanoma, and lung cancer.
And the path ahead is clear: expand trials, improve access, and scale personalized mRNA cancer vaccines globally.
But none of this will be possible without continued investment, public support, and ethical innovation. From biotech labs to regulatory agencies, and from researchers to patients, the next few years will determine whether this powerful technology becomes the new cornerstone of cancer care.
💬 If we could beat COVID in a year using mRNA, can cancer be next?
This is more than just a technological breakthrough—it’s a turning point in human health. The future of mRNA cancer treatment is not only about treating disease. It’s about transforming hope into medicine—one person, one vaccine, one cure at a time.
🧬 Fascinated by cutting-edge breakthroughs in cancer and biotech?
Explore more game-changing innovations reshaping the future of medicine:
-
⚛️ Terbium-161: Advancing Nuclear Cancer Therapy – Discover how this emerging radiopharmaceutical could redefine prostate cancer treatment.
-
🦴 Hydroxyapatite 2040 – A glimpse into the future of limb and dental regeneration using bioactive scaffolds.
-
💊 Methylene Blue: Bridging Medicine and Future Technology – From neuroprotection to anti-aging, explore the surprising modern uses of this century-old compound.
-
🤖 The Nanobots Cancer Cure – Learn how microscopic machines are being engineered to target and destroy cancer at the cellular level.
Click a title to continue your journey into the future of health innovation.